Additions to the Rust Fungi of South Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rust Disease of Water Willow Intercepted in Import Plant Quarantine in Japan
RES.BULL.PL.PROT.JAPAN No. 41: 59~64(2005) Short Communication Rust Disease of Water Willow Intercepted in Import Plant Quarantine in Japan Yoichi MOTOKURA, Masayoshi NAGASE*, Akihiro OOI**, Koshi UEDA, and SHIGERU KIMURA Research Division, Yokohama Plant Protection Station 1-16-10, Shin-yamashita, Naka-ku, Yokohama 231- 0801, Japan. * Nagoya Airport Branch, Nagoya Plant Protection Station ** Nagoya Plant Protection Station Abstract: A rust disease on water willow(Justicia gendarussa Burm f.)was found at an import plant quar- antine inspection at Nagoya airport, in January, 2002. The causal rust fungus was identified with Puccinia thwaitesii Berk., based on it's morphology and the results of inoculation experiments. This is the first report on the interception of rust disease of water willow caused by P. thwaitesii at import plant quarantine inspection in Japan. Key words: rust, water willow, Justicia gendarussa, Puccinia thwaitesii Introduction Water willow(Justicia gendarussa Burm f..)is a perennial shrub native to the tropical and sub- tropical zones of the Asia, and it belongs to Acanthaceae(Editorial Committee of the Flora of Taiwan,1998). In the Southeast Asia, this plant is utilized as a raw material of Chinese medicine, or as a medicine for rheumatism(IWATSUKI et. al. ed., 1997). In our country, water willow is introduced and used as an ornamental plant, mainly for indoor. In January 2002, potted plants of water willow infected with a rust disease were found at an import plant quarantine inspection at Komaki(Nagoya international airport)in Japan. They were plants imported from Thailand for use as the ornamental foliage. -

DPR Journal 2016 Corrected Final.Pmd
Bul. Dept. Pl. Res. No. 38 (A Scientific Publication) Government of Nepal Ministry of Forests and Soil Conservation Department of Plant Resources Thapathali, Kathmandu, Nepal 2016 ISSN 1995 - 8579 Bulletin of Department of Plant Resources No. 38 PLANT RESOURCES Government of Nepal Ministry of Forests and Soil Conservation Department of Plant Resources Thapathali, Kathmandu, Nepal 2016 Advisory Board Mr. Rajdev Prasad Yadav Ms. Sushma Upadhyaya Mr. Sanjeev Kumar Rai Managing Editor Sudhita Basukala Editorial Board Prof. Dr. Dharma Raj Dangol Dr. Nirmala Joshi Ms. Keshari Maiya Rajkarnikar Ms. Jyoti Joshi Bhatta Ms. Usha Tandukar Ms. Shiwani Khadgi Mr. Laxman Jha Ms. Ribita Tamrakar No. of Copies: 500 Cover Photo: Hypericum cordifolium and Bistorta milletioides (Dr. Keshab Raj Rajbhandari) Silene helleboriflora (Ganga Datt Bhatt), Potentilla makaluensis (Dr. Hiroshi Ikeda) Date of Publication: April 2016 © All rights reserved Department of Plant Resources (DPR) Thapathali, Kathmandu, Nepal Tel: 977-1-4251160, 4251161, 4268246 E-mail: [email protected] Citation: Name of the author, year of publication. Title of the paper, Bul. Dept. Pl. Res. N. 38, N. of pages, Department of Plant Resources, Kathmandu, Nepal. ISSN: 1995-8579 Published By: Mr. B.K. Khakurel Publicity and Documentation Section Dr. K.R. Bhattarai Department of Plant Resources (DPR), Kathmandu,Ms. N. Nepal. Joshi Dr. M.N. Subedi Reviewers: Dr. Anjana Singh Ms. Jyoti Joshi Bhatt Prof. Dr. Ram Prashad Chaudhary Mr. Baidhya Nath Mahato Dr. Keshab Raj Rajbhandari Ms. Rose Shrestha Dr. Bijaya Pant Dr. Krishna Kumar Shrestha Ms. Shushma Upadhyaya Dr. Bharat Babu Shrestha Dr. Mahesh Kumar Adhikari Dr. Sundar Man Shrestha Dr. -

Colours in Nature Colours
Nature's Wonderful Colours Magdalena KonečnáMagdalena Sedláčková • Jana • Štěpánka Sekaninová Nature is teeming with incredible colours. But have you ever wondered how the colours green, yellow, pink or blue might taste or smell? What could they sound like? Or what would they feel like if you touched them? Nature’s colours are so wonderful ColoursIN NATURE and diverse they inspired people to use the names of plants, animals and minerals when labelling all the nuances. Join us on Magdalena Konečná • Jana Sedláčková • Štěpánka Sekaninová a journey to discover the twelve most well-known colours and their shades. You will learn that the colours and elements you find in nature are often closely connected. Will you be able to find all the links in each chapter? Last but not least, if you are an aspiring artist, take our course at the end of the book and you’ll be able to paint as exquisitely as nature itself does! COLOURS IN NATURE COLOURS albatrosmedia.eu b4u publishing Prelude Who painted the trees green? Well, Nature can do this and other magic. Nature abounds in colours of all shades. Long, long ago people began to name colours for plants, animals and minerals they saw them in, so as better to tell them apart. But as time passed, ever more plants, animals and minerals were discovered that reminded us of colours already named. So we started to use the names for shades we already knew to name these new natural elements. What are these names? Join us as we look at beautiful colour shades one by one – from snow white, through canary yellow, ruby red, forget-me-not blue and moss green to the blackest black, dark as the night sky. -

Rust-Red Stringy Rot and Red Heart Rot Indian Paint Fungus and Bleeding Stereum in Firs
Rust-Red Stringy Rot and Red Heart Rot Indian paint fungus and bleeding Stereum in firs Pathogen—Rust-red stringy rot is caused by Echinodontium tinctorium. It is one of the few pathogens that has a common name—Indian paint fungus. Red heart rot is caused by Stereum (Haematostereum) sanguinolentum, also known as the bleeding Stereum. Hosts—Indian paint fungus attacks firs, hemlocks, and less commonly other species in western NorthAmerica, but it is primarily found on white fir in the Rocky Mountain Region. Bleeding Stereum occurs primarily on subal- pine fir and Engelmann spruce in this Region, though it can also attack other conifers. Signs and Symptoms—Indian paint fungus produces conks frequently at branch stubs, at wounds, and even inside hollow stems. The conk is hard and woody, hoof-shaped, and perennial (figs. 1-3). The upper surface is blackish and rough with crevices. The lower surface is grey-brown with hard, blunt, thick teeth. The inside is brilliant brick- or rust-red, but it fades over time after exposure. This tissue was ground into a powder and used as paint by some Native Americans, giving the fungus its name. The only other indicator of this disease is punk knots known as “rusty knots.” Although there is no swelling as with punk knots caused by Porodaedalea pini, the interior of the knot shows the rust-red color characteristic of the decay. Bleeding Stereum fruits frequently on logs and slash, but infrequently on live trees. Conks are small, thin, incon- spicuous, and leathery (fig. 4). Portions are appressed to the bark, often with a projecting cap. -

Color Chart.Pdf
® Finishing Products Division of RPM Wood Finishes Group Inc. Color Chart The Original Touch Up Company™ Made in the USA Color Chart ® Finishing Products Division of RPM Wood Finishes Group, Inc. Index Aerosols 1-5 Ultra® Classic Toner & Tone Finish Toner 1-3 Colored Lacquer Enamel 3-5 Shadow Toner 5 Touch-Up Markers/Pencils 5-15 Ultra® Mark Markers 5-9 3 in 1 Repair Stick 9 Pro-Mark® Markers 9-10 Quik-Tip™ Markers 10-11 Background Marker Touch-Up & Background Marker Glaze Hang-Up 11-13 Artisan Glaze Markers 13 Vinyl Marker Glaze Hang-Up 14 Brush Tip Graining Markers 14 Accent Pencils 15 Blend-Its 15 Fillers 15-29 Quick Fill® Burn-In Sticks 15-16 Edging/Low Heat Sticks 16 E-Z Flow™ Burn-In Sticks 16-17 PlaneStick® Burn-In Sticks 17-18 Fil-Stik® Putty Sticks 18-25 Hard Fill & Hard Fill Plus 25-27 PermaFill™ 27 Epoxy Putty Sticks 27-28 Patchal® Puttys 28-29 Knot Filler 29 Fil-O-Wood™ Wood Putty Tubes 29 Color Replacement 30-31 Blendal® Sticks 30 Sand Thru Sticks 30-31 Blendal® Powder Stains 31 Bronzing Powders 31 Dye Stains 32 Ultra® Penetrating & Architectural Ultra® Penetrating Stain 32 Dye Concentrate 32 Pigmented Stains 32-34 Wiping Wood™, Architectural Wiping Stain & Wiping Wood™ Stain Aerosols 32-33 Designer Series Stain, Designer Series Radiant Stain 33-34 Glazes 34 Finisher’s Glaze™ Glazing Stain & Aerosols 34 Break-A-Way™ Glaze & Aerosols 34 Leather Repair 35-37 E-Z Flow™ Leather Markers 35 Leather/Vinyl Markers 35 Leather/Vinyl Fil Sticks 35-36 Leather Repair Basecoat Aerosols 36 Leather Repair Toner Aerosols 36 Leather Repair Color Adjuster Aerosols 37 Touch Up Pigment 37 Leather Refinishing 37 Base Coat 37 NOTE: COLORS ARE APPROXIMATE REPRESENTATIONS OF ACTUAL COLORS USING MODERN PROCESS TECHNIQUES. -

Current Status of Research on Rust Fungi (Pucciniales) in India
Asian Journal of Mycology 4(1): 40–80 (2021) ISSN 2651-1339 www.asianjournalofmycology.org Article Doi 10.5943/ajom/4/1/5 Current status of research on Rust fungi (Pucciniales) in India Gautam AK1, Avasthi S2, Verma RK3, Devadatha B 4, Sushma5, Ranadive KR 6, Bhadauria R2, Prasher IB7 and Kashyap PL8 1School of Agriculture, Abhilashi University, Mandi, Himachal Pradesh, India 2School of Studies in Botany, Jiwaji University, Gwalior, Madhya Pradesh, India 3Department of Plant Pathology, Punjab Agricultural University, Ludhiana, Punjab, India 4 Fungal Biotechnology Lab, Department of Biotechnology, School of Life Sciences, Pondicherry University, Kalapet, Pondicherry, India 5Department of Biosciences, Chandigarh University Gharuan, Punjab, India 6Department of Botany, P.D.E.A.’s Annasaheb Magar Mahavidyalaya, Mahadevnagar, Hadapsar, Pune, Maharashtra, India 7Department of Botany, Mycology and Plant Pathology Laboratory, Panjab University Chandigarh, India 8ICAR-Indian Institute of Wheat and Barley Research (IIWBR), Karnal, Haryana, India Gautam AK, Avasthi S, Verma RK, Devadatha B, Sushma, Ranadive KR, Bhadauria R, Prasher IB, Kashyap PL 2021 – Current status of research on Rust fungi (Pucciniales) in India. Asian Journal of Mycology 4(1), 40–80, Doi 10.5943/ajom/4/1/5 Abstract Rust fungi show unique systematic characteristics among all fungal groups. A single species of rust fungi may produce up to five morphologically and cytologically distinct spore-producing structures thereby attracting the interest of mycologist for centuries. In India, the research on rust fungi started with the arrival of foreign visiting scientists or emigrant experts, mainly from Britain who collected fungi and sent specimens to European laboratories for identification. Later on, a number of mycologists from India and abroad studied Indian rust fungi and contributed a lot to knowledge of the rusts to the Indian Mycobiota. -
J9 Packline Report V8
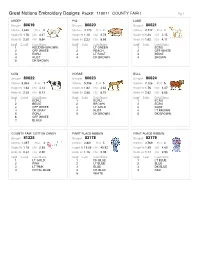
Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 1 SHEEP PIG LAMB Design# 80619Design# 80620Design# 80621 Stitches 4,622 #Col 5 Stitches 4,170 #Col 4 Stitches 2,747 #Col 4 Height IN 1.76 CM 4.47 Height IN 1.10 CM 2.79 Height IN 1.24 CM 3.15 Width IN 2.30 CM 5.66 Width IN 2.23 CM 5.66 Width IN 1.62 CM 4.11 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 REDDISH BROWN 1 LT GREEN 1 ECRU 2 OFF WHITE 2 PEACH 2 OFF WHITE 3 ECRU 3 LT RUST 3 BEIGE 4 RUST 4 DK BROWN 4 BROWN 5 DK BROWN COW HORSE BULL Design# 80622Design# 80623Design# 80624 Stitches 5,834 #Col 7 Stitches 5,756 #Col 5 Stitches 7,126 #Col 5 Height IN 1.63 CM 4.14 Height IN 1.82 CM 4.62 Height IN 1.76 CM 4.47 Width IN 2.35 CM 6.73 Width IN 2.65 CM 6.73 Width IN 2.62 CM 6.65 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 ECRU 1 ECRU 1 ECRU 2 BEIGE 2 BROWN 2 ECRU 3 OFF WHITE 3 LT GOLD 3 RUST 4 DK GRAY 4 RUST 4 LT BROWN 5 ECRU 5 DK BROWN 5 DK BROWN 6 OFF WHITE 7 BLACK 'COUNTY FAIR' COTTON CANDY FIRST PLACE RIBBON FIRST PLACE RIBBON Design# 81325Design# 83178Design# 83179 Stitches 1,397 #Col 4 Stitches 3,801 #Col 5 Stitches 3,769 #Col 4 Height IN 1.16 CM 2.95 Height IN 18.08 CM 45.92 Height IN 1.89 CM 4.80 Width IN 2.34 CM 2.95 Width IN 1.16 CM 2.95 Width IN 1.14 CM 2.90 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 LT GOLD 1 DK BLUE 1 LT BLUE 2 PINK 2 LT BLUE 2 BLUE 3 LT PINK 3 BLUE 3 DK BLUE 4 ROYAL BLUE 4 DK BLUE 4 RED 5 WHITE Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 2 FIRST PLACE -

Common Asian Rubus Rust-Hamaspora Acutissima This Is One of Numerous Rust Fungi That Attack Species of Rubus and Are Widespread in Asia
U.S. Department of Agriculture, Agricultural Research Service Systematic Mycology and Microbiology Laboratory - Invasive Fungi Fact Sheets Common Asian Rubus Rust-Hamaspora acutissima This is one of numerous rust fungi that attack species of Rubus and are widespread in Asia. They do not appear to be very damaging. The elongated teliospores of species of Hamaspora are distinctive. Hamaspora acutissima P. Syd. & Syd. 1912 Spermogonia and aecia unknown. Uredinia hypophyllous (on lower surface of leaves), sparse, minute, orange-yellow; urediniospores globose, subglobose or ellipsoid, 18-28 × 13-20 µm, walls yellow, echinulate, 1-2 µm thick, germ pores 5-6, scattered. Telia amphigenous (on upper and lower side of leaves), mainly hypophyllous, filiform, up to 8 mm, white or pale yellow; teliospores cylindrical or obclavate to acicular, 3- to 6-celled (mostly 4- to 5-celled) constricted at septum, 120-240 µm ×, 16-25 µm, apical cells long, 20-35 µm long, acuminate at apices, walls yellow, smooth, germ pores 1 per cell; pedicels long, persistent, up to 800 µm long, 10-15 µm wide. See Hiratsuka et al. (1992) and Monoson (1969) for a more detailed description. Host Range: Uredinial and telial state: Rubus alceifolius Poir. Rubus calycinoides Hayata Rubus elmeri Focke Rubus formosensis Kuntze Rubus fraxinifolius Poir. Rubus glomeratus Blume Rubus laciniatostipulatus Hayata & Koidz. Rubus moluccanus L. Rubus nantoensis Hayata Rubus nesiotes Focke & Focke Rubus niveus Thunb. Rubus parkeri Hance Rubus pectinellus Maxim. Rubus pyrifolius Sm. Rubus rolfei S. Vidal Rubus rosaefolius Sm. Rubus setchuenensis Bureau & Franch. Rubus sp. Rubus swinhoei Hance Rubus tagallus Cham. & Schlecht. Geographic distribution: Australia, Far East Asia (China, Indonesia, Japan, New Caledonia, Papua-New Guinea, Philippines, Taiwan) Notes: This is one of the most common species of Hamaspora on Rubus and is quite distinctive in having very long, threadlike teliospores. -

Earthy Browns & Soft Neutrals WOMAN COLLECTION F/W 20-21
Authentic product Designed in Belgium Made in Peru Earthy browns & Soft neutrals WOMAN COLLECTION F/W 20-21 GREY CAMEL BLACK RUST NAVY JACOBS 70% baby alpaca 30% mulberry silk XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru GREY CAMEL BLACK RUST NAVY CELINE 70% baby alpaca 30% mulberry silk XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru GREY CAMEL BLACK RUST NAVY PAULINE 70% baby alpaca 30% mulberry silk XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL PEACH GLACIAR NAVY BRANDY WINE HONEY CORAL AIR BLACK FELICE 60% baby alpaca 5% merino wool 35% PA XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL PEACH GLACIAR NAVY BRANDY WINE HONEY CORAL AIR BLACK ANDINO stripe & plain colour version 60% baby alpaca 5% merino wool 35% PA XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL PEACH GLACIAR NAVY BRANDY WINE HONEY CORAL AIR BLACK PISCO 60% baby alpaca 5% merino wool 35% PA XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL PEACH GLACIAR NAVY BRANDY WINE HONEY CORAL AIR BLACK SURI 75% baby alpaca 25% acrylic XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL PEACH GLACIAR NAVY BRANDY WINE HONEY CORAL AIR BLACK SOL 75% baby alpaca 25% acrylic XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL NAVY WINE BLACK PINE RUST COLCA 70% baby alpaca 7% merino wool 23% PA XS-S-M-L-XL Authentic product Designed in Belgium Made in Peru ECRU GREY CAMEL NAVY -

Orange | Colour Choices Booklet
orange Positive – Orange is Happy, fun, sassy, playful, optimistic and energetic. Orange Orange looks great with all forms of blue – turquoise, warm, passionate, sensual is such an irrepressibly cheerful colour that it makes most teal, ice-blue, true blue, slate blue – as blue sits opposite and fun. The brighter of us smile. Some people are not so keen on its in-your- orange on the colour wheel. As with any scheme based on shades are cheerful and face exuberance, but orange as an interior colour is very the use of complementary colours, make sure you get the tend to stimulate the useful, particularly in burnt or terracotta tones, like Resene saturation and balance of colours right. appetite, making them Fire. Because orange is such a strong colour, it has often ideal for kitchens and been reserved for use as an accent for feature walls and Orange has a cool retro appeal that goes with mid- dining rooms, where they splashbacks. But there are many ways to include its century-style interiors. It’s also a good colour to use in also create a comfortable, brightness in your home. children’s rooms if you don’t want to fall into the unisex cosy atmosphere. stereotypes of pink or blue. Make sure you tone it down a From peach to pumpkin little though, or orange’s stimulating power will keep the Negative – Feelings of Orange is a hugely adaptable colour: it starts at soft peach, kiddies awake. deprivation, most likely like Resene Porsche, then ranges through coral, melon to You can go all out in the kitchen though, where orange’s when orange is combined carrot, then goes on to deeper shades of terracotta and warmth can be channelled to create a inviting social with black. -

Pple, Crabapple, Hawthorn, and Juniper Disorder: Cedar-Rust Complex AG.L
A2598 Disease pple, crabapple, hawthorn, and juniper disorder: Cedar-rust complex AG.L. WORF and M.F. HEIMANN Cedar-rust complex is caused by spots also are evident on the leaves’ On fruit, cedar-apple rust causes a fungus that requires two hosts— undersurface, but remain yellow orange spots similar to those found juniper plus apple, crabapple, rather than orange. The lesions vary on leaves, but the spots are usually hawthorn, or quince—to complete its in size depending on the variety of much larger. The lesions are usually life cycle. This disease is found any- tree and the number of spots present found near the bottom of the fruit. where that junipers (also known as on a single leaf. The affected leaf The normal light-green coloring of red cedars) grow and can cause con- tissue swells but rarely dies. Bulges young fruit becomes darker around siderable damage when both hosts appear on the underside of the spots. the border of the affected area. The are grown near each other. The bulges later develop cylindrical cylindrical fruiting bodies that are tubes that are open on the undersur- found on leaves and twigs rarely Symptoms and effects face and contain light-brown spores. appear on the fruit, but when present Deciduous hosts In July and August, the infected they are usually in a circle outside the Cedar-apple rust symptoms leaves may drop. Defoliation is more dark centers of the spots. occur primarily on leaves, although severe during dry summers. they may appear on twigs and fruit. On twigs, the cedar-apple rust Small, pale yellow spots appear on first appears on the current season’s the leaves’ upper surface in May. -

W68 Killrust Colour Guide-6.Indd 1-3 27/3/12 10:47:21 AM Killrust Superior Protection Epoxy Enamel Killrust Superior Protection Epoxy Enamel
Killrust System Guide Killrust – The superior system for the protection of metal surfaces The Killrust system makes painting metal an easy three step process. The system is specially To rejuvenate, beautify, and protect your metal surfaces, we recommend you follow the step by formulated to rejuvenate, beautify, and protect your metal surfaces. step Killrust system shown below. Step 1: Prepare The Killrust range of preparatory products provide superior solutions for preparing rusted metal, clean steel, and difficult to reach surfaces. SURFACE Killrust Rust-Eeter® Killrust Cold Galvit Killrust Fishoilene Killrust Metal Prep PREPARE PRIME TOPCOAT TYPE/CONDITION Sand surface lightly until gloss is removed; remove Previously painted in dust. Any mould spots Apply Killrust Superior No priming required. good condition. must be treated with Protection Epoxy Enamel. bleach, then rinsed with clean water. • Converts rust to stop corrosion • Zinc-rich cold galvanising primer • An anti-corrosive protective • Kills & neutralises rust, for long-term, extra heavy duty fluid for rusted steel where normal exposing a clean surface for • Provides a suitable surface corrosion protection surface preparation is difficult painting for subsequent priming and painting • Encapsulates the metal in a zinc film • Ideal for hard to reach areas - • Improves adhesion and cracks, crevices, along pipes, corrosion resistance • For use on wrought iron and • Ideal for use on clean, rust free Steel or wrought iron. Clean surface to remove Apply Killrust Metal Apply Killrust Superior nuts, bolts, hinges and welds New, unpainted. oil, grease and mill-scale. Primer. Protection Epoxy Enamel. ferrous metal surfaces steel in highly corrosive • For use on mildly rusted steel environments such as coastal regions and other ferrous metals, • Not for use on galvanised iron • For use on steel handrails, metal gates, metal railings balustrading and other structural and metal lattice work steelwork • Not for use on galvanised iron Wire brush, scrape or sand surface to remove Steel or wrought iron.