Life Cycle Studies of Some Antarctic Mites and Description of a New Species, Protereunetes Paulinae Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biennial Review 1969/70 Bedford Institute Dartmouth, Nova Scotia Ocean Science Reviews 1969/70 A
(This page Blank in the original) ii Bedford Institute. ii Biennial Review 1969/70 Bedford Institute Dartmouth, Nova Scotia Ocean Science Reviews 1969/70 A Atlantic Oceanographic Laboratory Marine Sciences Branch Department of Energy, Mines and Resources’ B Marine Ecology Laboratory Fisheries Research Board of Canada C *As of June 11, 1971, Department of Environment (see forward), iii (This page Blank in the original) iv Foreword This Biennial Review continues our established practice of issuing a single document to report upon the work of the Bedford Institute as a whole. A new feature introduced in this edition is a section containing four essays: The HUDSON 70 Expedition by C.R. Mann Earth Sciences Studies in Arctic Marine Waters, 1970 by B.R. Pelletier Analysis of Marine Ecosystems by K.H. Mann Operation Oil by C.S. Mason and Wm. L. Ford They serve as an overview of the focal interests of the past two years in contrast to the body of the Review, which is basically a series of individual progress reports. The search for petroleum on the continental shelves of Eastern Canada and Arctic intensified considerably with several drilling rigs and many geophysical exploration teams in the field. To provide a regional depository for the mandatory core samples required from all drilling, the first stage of a core storage and archival laboratory was completed in 1970. This new addition to the Institute is operated by the Resource Administration Division of the Department of Energy, Mines & Resources. In a related move the Geological Survey of Canada undertook to establish at the Institute a new team whose primary function will be the stratigraphic mapping of the continental shelf. -

Explorer's Gazette
EEXXPPLLOORREERR’’SS GGAAZZEETTTTEE Published Quarterly in Pensacola, Florida USA for the Old Antarctic Explorers Association Uniting All OAEs in Perpetuating the Memory of United States Involvement in Antarctica Volume 15, Issue 1 Old Antarctic Explorers Association, Inc Jan-Mar 2015 —Photo by Mary Stortstrom John Strider With His Print of the Famous Landing FIRST AMERICAN TO SET FOOT ON THE GEOGRAPHIC SOUTH POLE RECALLS DETAILS OF HIS EXPERIENCE Story by Mary Stortstrom The then-25-year-old Strider joined the newly The Journal Martinsburg West Virginia commissioned Air Development Squadron Six (VX-6) for 15 March 2015 the military mission, Operation Deep Freeze, a mission to Edited by Billy-Ace Baker support scientist during the International Geophysical Year. From Tennessee, Strider and the other men in VX-6 flew n the comfort of the Elmcroft senior living center, 85-year- to Alameda, California, from there to Hawaii, then Canton, I old John Philip Strider, retired US Navy chief petty the Christmas Islands, Fiji, and finally Christchurch, New officer, can still recall every detail of the historic Operation Zealand, the unit's last stop before flying to Antarctica. Deep Freeze II expedition to Antarctica. “Getting there was the big thing,” Strider said. “We had Strider said he remembers sitting and sharing a meal with to stop for gas, but we never stopped for crew rest because a few other members of his unit stationed in Tennessee in we had a big enough crew that we just slept on the plane and May of 1955 and hearing that the Bureau of Navy Personnel relieved each other of duties.” was looking for volunteers to go to Antarctica. -

Explorer's Gazette
EEXXPPLLOORREERR’’SS GAZETTE GAZETTE Published Quarterly in Pensacola, Florida USA for the Old Antarctic Explorers Association Uniting All OAEs in Perpetuating the Memory of United States Involvement in Antarctica Volume 13, Issue 2 Old Antarctic Explorers Association, Inc Apr-Jun 2013 —Photo by Elaine Hood/NSF Brandon “Shaggy” Neahusan explains the vision for rebuilding McMurdo Station over the next 15 years. Antarctic Deep Freeze Association Reunion New Orleans 2013 by Elaine Hood working in McMurdo was the last year the Navy was there, Easy Fun in the Big Easy 1998. I’ve been a member of ADFA since learning about its existence almost ten years ago. I have not missed an ADFA The Big Easy, New Orleans, was the destination of dozens reunion since my first one and always look forward to seeing of Deep Freeze veterans June 18–21. And the living was familiar faces and laughing at good stories. easy due to the prime location of the Hyatt Hotel in the French Quarter, as it was located right on Bourbon Street. Reunion The nightlife on Bourbon Street never stops and it is always Bill Stroup and his son Robert were our hosts for the worth a walk just to see what trouble you need to stay away biennial Antarctic Deep Freeze Association reunion. Robert from. kept the bar well stocked in the hospitality room that always My name is Elaine Hood and I work for the civilian serves as the central meeting point. contractor to the US Antarctic Program. My first year Continued on page 4. E X P L O R E R ‘ S G A Z E T T E V O L U M E 13, I S S U E 2 A P R J U N 2 0 1 3 P R E S I D E N T ’ S C O R N E R Laura Snow—OAEA President FELLOW OAEA MEMBERS: In this first letter to you from the President’s Corner I am providing you with information regarding recent decisions by the board of directors. -

Insects and Related Arthropods Associated with of Agriculture
USDA United States Department Insects and Related Arthropods Associated with of Agriculture Forest Service Greenleaf Manzanita in Montane Chaparral Pacific Southwest Communities of Northeastern California Research Station General Technical Report Michael A. Valenti George T. Ferrell Alan A. Berryman PSW-GTR- 167 Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 9470 1 -0245 Abstract Valenti, Michael A.; Ferrell, George T.; Berryman, Alan A. 1997. Insects and related arthropods associated with greenleaf manzanita in montane chaparral communities of northeastern California. Gen. Tech. Rep. PSW-GTR-167. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Dept. Agriculture; 26 p. September 1997 Specimens representing 19 orders and 169 arthropod families (mostly insects) were collected from greenleaf manzanita brushfields in northeastern California and identified to species whenever possible. More than500 taxa below the family level wereinventoried, and each listing includes relative frequency of encounter, life stages collected, and dominant role in the greenleaf manzanita community. Specific host relationships are included for some predators and parasitoids. Herbivores, predators, and parasitoids comprised the majority (80 percent) of identified insects and related taxa. Retrieval Terms: Arctostaphylos patula, arthropods, California, insects, manzanita The Authors Michael A. Valenti is Forest Health Specialist, Delaware Department of Agriculture, 2320 S. DuPont Hwy, Dover, DE 19901-5515. George T. Ferrell is a retired Research Entomologist, Pacific Southwest Research Station, 2400 Washington Ave., Redding, CA 96001. Alan A. Berryman is Professor of Entomology, Washington State University, Pullman, WA 99164-6382. All photographs were taken by Michael A. Valenti, except for Figure 2, which was taken by Amy H. -
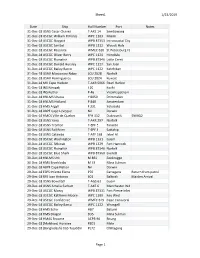
1/23/2019 Sheet1 Page 1 Date Ship Hull Number Port Notes 31-Dec
Sheet1 1/23/2019 Date Ship Hull Number Port Notes 31-Dec-18 USNS Cesar Chavez T-AKE 14 Sembawang 31-Dec-18 USCGC William R Flores WPC 1103 Miami 31-Dec-18 USCGC Skipjack WPB 87353 Intracoastal City 31-Dec-18 USCGC Sanibel WPB 1312 Woods Hole 31-Dec-18 USCGC Resolute WMEC 620 St Petersburg FL 31-Dec-18 USCGC Oliver Berry WPC 1124 Honolulu 31-Dec-18 USCGC Flyingfish WPB 87346 Little Creek 31-Dec-18 USCGC Donald Horsley WPC 1127 San Juan 31-Dec-18 USCGC Bailey Barco WPC 1122 Ketchikan 31-Dec-18 USAV Missionary Ridge LCU 2028 Norfolk 31-Dec-18 USAV Hormigueros LCU 2024 Kuwait 31-Dec-18 MV Cape Hudson T-AKR 5066 Pearl Harbor 31-Dec-18 INS Nirupak J 20 Kochi 31-Dec-18 INS Kuthar P 46 Visakhapatnam 31-Dec-18 HNLMS Urania Y 8050 Drimmelen 31-Dec-18 HNLMS Holland P 840 Amsterdam 31-Dec-18 HMS Argyll F 231 Yokosuka 31-Dec-18 ABPF Cape Leveque Nil Darwin 30-Dec-18 HMCS Ville de Quebec FFH 332 Dubrovnik SNMG2 30-Dec-18 USNS Yano T-AKR 297 Norfolk 30-Dec-18 USNS Trenton T-EPF 5 Taranto 30-Dec-18 USNS Fall River T-EPF 4 Sattahip 30-Dec-18 USNS Catawba T-ATF 168 Jebel Ali 30-Dec-18 USCGC Washington WPB 1331 Guam 30-Dec-18 USCGC Sitkinak WPB 1329 Fort Hancock 30-Dec-18 USCGC Flyingfish WPB 87346 Norfolk 30-Dec-18 USCGC Blue Shark WPB 87360 Everett 30-Dec-18 HNLMS Urk M 861 Zeebrugge 30-Dec-18 HMS Brocklesby M 33 Mina Sulman 30-Dec-18 ABPF Cape Nelson Nil Darwin 29-Dec-18 ESPS Infanta Elena P76 Cartagena Return from patrol 29-Dec-18 RFS Ivan Antonov 601 Baltiysk Maiden Arrival 29-Dec-18 USNS Bowditch T-AGS 62 Guam 29-Dec-18 USNS Amelia Earhart T-AKE 6 -

Faunistic Analysis of Soil Mites in Coffee Plantation
International Journal of Environmental & Agriculture Research (IJOEAR) ISSN:[2454-1850] [Vol-4, Issue-3, March- 2018] Faunistic Analysis of Soil Mites in Coffee Plantation Patrícia de Pádua Marafeli1, Paulo Rebelles Reis2, Leopoldo Ferreira de Oliveira Bernardi3, Pablo Antonio Martinez4 1Universidade Federal de Lavras - UFLA, Lavras, MG, Brazil. Entomology Postgraduate Program. 2Empresa de Pesquisa Agropecuária de Minas Gerais - EPAMIG Sul/EcoCentro, Lavras, MG, Brazil. CNPq Researcher. 3Universidade Federal de Lavras - UFLA - Departamento de Biologia/DBI – Setor de Ecologia Aplicada, Lavras, MG. Brazil. CAPES / PNPD scholarship holder. 4Universidad Nacional de La Plata, La Plata, Argentina. Abstract ─ The soil-litter system is the natural habitat for a wide variety of organisms, microorganisms and invertebrates, with differences in size and metabolism, which are responsible for numerous functions. The soil mesofauna is composed of animals of body diameter between 100 μm and 2 mm, consisting of the groups Araneida, Acari, Collembola, Hymenoptera, Diptera, Protura, Diplura, Symphyla, Enchytraeidae (Oligochaeta), Isoptera, Chilopoda, Diplopoda and Mollusca. These animals, extremely dependent on humidity, move in the pores of the soil and at the interface between the litter and the soil. The edaphic fauna, besides having a great functional diversity, presents a rich diversity of species. As a result, these organisms affect the physical, chemical and, consequently, the biological factors of the soil. Therefore, the edaphic fauna and its activities are of extreme importance so that the soil is fertile and can vigorously support the vegetation found there, being spontaneous or cultivated. The composition, distribution and density of the edaphic acarofauna varies according to the soil depth, mites size, location and the season of the year. -

Explorer's Gazette Listed the Thank You for the Email
EEXXPPLLOORREERR’’SS GAZETTE GAZETTE Published Quarterly in Pensacola, Florida USA for the Old Antarctic Explorers Association Uniting All OAEs in Perpetuating the History of U.S. Navy Involvement in Antarctica Volume 9, Issue 2 Old Antarctic Explorers Association, Inc Apr-Jun 2009 South Pole Station 10-Meter Telescope Facility 2009 Antarctic Deep Freeze Association Reunion Madison Wisconsin by Elaine Hood HE 2009 ANTARCTIC DEEP FREEZE ASSOCIATION Ed, and his wife, Rosanne kicked off the event with a (ADFA) reunion was held in Middleton, Wisconsin, traditional Badgerland tailgate feed hosted at their lovely T 2–4 June, hosted by Dr. Ed Ehrlich. Ed was the Middleton home for a horde of early arrivals. Al Hisey Medical Officer at Little America V, Deep Freeze I. (McM, DF-I&II) and Dave Grisez (McM, DF-I&II) both He was a Professor of Medicine at the University of were immediately drafted to man the grilling of the burgers Wisconsin for 35 years and is now a Professor Emeritus. He and brats while old friends caught up with each other and continues to practice his specialty in endocrinology as a new acquaintances were made. volunteer at a free clinic in Middleton. See: ADFA Reunion On page 4. E X P L O R E R ‘ S G A Z E T T E V O L U M E 9, I S S U E 2 A P R J U N 2 0 0 9 P R E S I D E N T ’ S C O R N E R James “Jim Da Retired Cop” Heffel—OAEA President TO ALL OAEs—It would seem that I have caused confusion with my offer of a free airline ticket to the 2010 reunion. -

Explorer's Gazette
EEXXPPLLOORREERR’’SS GAZETTE GAZETTE Published Quarterly in Pensacola, Florida USA for the Old Antarctic Explorers Association Uniting All OAEs in Perpetuating the History of U.S. Navy Involvement in Antarctica Volume 7, Issue 4 Old Antarctic Explorers Association, Inc Oct-Dec 2007 Photo by Margaret Adams South Pole Station Christmas Tree — 25 December 2007 F U N A N D G A M E S A T T H E S O U T H P O L E Compiled by Billy-Ace Baker HE ABOVE PHOTO WAS TAKEN BY A YOUNG LADY like a logical step after spending much of the season fixing FROM HOLDEN MAINE WHO SPENT THE ASUTRAL them”. summer working at South Pole Station. Margaret, Meg was scheduled to leave the South Pole on one of the T aka, Meg is now working as an Operations General last flights out in February. However, she has volunteered to Assistant (OPS GA). According to her in an email message participate in the “Extended Season”, and if selected, she “I lucked out; working as a cross between the Heavy Shop will spend several more weeks as the cook at the Marble Ops GA and the Traditional Ops GA has given me the Point helicopter facility. opportunity to work all over South Pole Station, while still In spite of her full schedule as a “humble” GA, Meg also forming specific ties with the mechanics in the Heavy writes stories about life at the South Pole that appear weekly Shop”. Meg hopes to come back next year and work for in the Bangor Maine Daily News. -

CURRICULUM VITAE 10 August 2015
IAN W. D. DALZIEL B.SC., PH.D., D.SC. (Hon), FRSE CURRICULUM VITAE 10 August 2015 PERSONAL ..................................................................................................................................................... 1 EDUCATION ................................................................................................................................................... 1 AWARDS AND DISTINCTIONS ........................................................................................................................ 1 ACADEMIC POSITIONS ................................................................................................................................... 1 ADMINISTRATIVE POSITIONS ........................................................................................................................ 2 MAIN FIELDS OF RESEARCH INTEREST ......................................................................................................... 2 ELECTIONS TO FELLOWSHIP .......................................................................................................................... 2 EDITORIAL RESPONSIBILITIES ....................................................................................................................... 2 LEADERSHIP OF MAJOR INTERNATIONAL RESEARCH PROJECTS .................................................................. 2 CONVENER, INTERNATIONAL WORKSHOPS AND CONFERENCES ................................................................. 2 PROFESSORSHIPS, LECTURESHIPS, AND -

1 Appendix 3. Rouge National Park Taxonomy Report
Appendix 3. Rouge National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Amaurobiidae Callobius Callobius bennetti Anyphaenidae Anyphaena Anyphaena celer Anyphaena pectorosa Hibana Hibana gracilis Wulfila Wulfila saltabundus Araneidae Acanthepeira Acanthepeira stellata Araneus Araneus diadematus Araneus trifolium Eustala Eustala anastera Eustala emertoni Gea Gea heptagon Hypsosinga Hypsosinga rubens Mangora Mangora gibberosa Mangora placida Neoscona Neoscona arabesca Zygiella Zygiella atrica Clubionidae Clubiona Clubiona abboti Clubiona johnsoni Clubiona kastoni Clubiona obesa Clubiona pygmaea Dictynidae Cicurina Cicurina itasca 1 Cicurina pallida Dictyna Dictyna volucripes Emblyna Emblyna decaprini Emblyna hentzi Emblyna sublata Gnaphosidae Drassyllus Drassyllus depressus Drassyllus niger Gnaphosa Gnaphosa parvula Zelotes Zelotes hentzi Hahniidae Neoantistea Neoantistea gosiuta Linyphiidae Agyneta Agyneta serrata Agyneta sheffordiana Centromerus Centromerus sylvaticus Ceraticelus Ceraticelus atriceps Ceraticelus fissiceps Ceraticelus laticeps Ceraticelus similis Ceratinella Ceratinella brunnea Collinsia Collinsia plumosa Diplostyla Diplostyla concolor Erigone Erigone autumnalis Frontinella Frontinella communis Grammonota Grammonota angusta Hypselistes Hypselistes florens Lepthyphantes Lepthyphantes leprosus Mermessus Mermessus trilobatus Neriene Neriene radiata Neriene variabilis 2 Walckenaeria Walckenaeria atrotibialis Walckenaeria directa Wubana -

Diversity and Distribution of Mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago
Article Diversity and Distribution of Mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago Anna Seniczak 1,*, Stanisław Seniczak 2, Marla D. Schwarzfeld 3 and Stephen J. Coulson 4,5 and Dariusz J. Gwiazdowicz 6 1 Department of Natural History, University Museum of Bergen, University of Bergen, Postboks 7800, 5020 Bergen, Norway 2 Department Evolutionary Biology, Faculty of Biological Sciences, Kazimierz Wielki University, J.K. Chodkiewicza 30, 85-064 Bydgoszcz, Poland; [email protected] 3 Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-food Canada, 960 Carling Avenue, Ottawa, ON K1A 0C6, Canada; [email protected] 4 Swedish Species Information Centre, Swedish University of Agricultural Sciences, SLU Artdatabanken, Box 7007, 75007 Uppsala, Sweden; [email protected] 5 Department of Arctic Biology, University Centre in Svalbard, P.O. Box 156, 9171 Longyearbyen, Svalbard, Norway 6 Faculty of Forestry, Poznań University of Life Sciences, Wojska Polskiego 71c, 60-625 Poznań, Poland; [email protected] * Correnspondence: [email protected] Received: 21 July 2020; Accepted: 19 August 2020; Published: 25 August 2020 Abstract: Svalbard is a singular region to study biodiversity. Located at a high latitude and geographically isolated, the archipelago possesses widely varying environmental conditions and unique flora and fauna communities. It is also here where particularly rapid environmental changes are occurring, having amongst the fastest increases in mean air temperature in the Arctic. One of the most common and species-rich invertebrate groups in Svalbard is the mites (Acari). We here describe the characteristics of the Svalbard acarofauna, and, as a baseline, an updated inventory of 178 species (one Ixodida, 36 Mesostigmata, 43 Trombidiformes, and 98 Sarcoptiformes) along with their occurrences. -

A Catalog of Acari of the Hawaiian Islands
The Library of Congress has catalogued this serial publication as follows: Research extension series / Hawaii Institute of Tropical Agri culture and Human Resources.-OOl--[Honolulu, Hawaii]: The Institute, [1980- v. : ill. ; 22 cm. Irregular. Title from cover. Separately catalogued and classified in LC before and including no. 044. ISSN 0271-9916 = Research extension series - Hawaii Institute of Tropical Agriculture and Human Resources. 1. Agriculture-Hawaii-Collected works. 2. Agricul ture-Research-Hawaii-Collected works. I. Hawaii Institute of Tropical Agriculture and Human Resources. II. Title: Research extension series - Hawaii Institute of Tropical Agriculture and Human Resources S52.5.R47 630'.5-dcI9 85-645281 AACR 2 MARC-S Library of Congress [8506] ACKNOWLEDGMENTS Any work of this type is not the product of a single author, but rather the compilation of the efforts of many individuals over an extended period of time. Particular assistance has been given by a number of individuals in the form of identifications of specimens, loans of type or determined material, or advice. I wish to thank Drs. W. T. Atyeo, E. W. Baker, A. Fain, U. Gerson, G. W. Krantz, D. C. Lee, E. E. Lindquist, B. M. O'Con nor, H. L. Sengbusch, J. M. Tenorio, and N. Wilson for their assistance in various forms during the com pletion of this work. THE AUTHOR M. Lee Goff is an assistant entomologist, Department of Entomology, College of Tropical Agriculture and Human Resources, University of Hawaii. Cover illustration is reprinted from Ectoparasites of Hawaiian Rodents (Siphonaptera, Anoplura and Acari) by 1. M. Tenorio and M. L.