Assessment of the Weed Control Programme on Raoul Island, Kermadec Group
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Furcraea Foetida
Furcraea foetida Mauritius hemp Furcraea foetida (L.) Haw. Syn. Agave foetida, Furcraea gigantea Family: Agavaceae Description: Long pointed leaves, to 8 ft long by 8 inches wide, light green, succulent, arranged densely around a short stem, a few widely spaced prickles on margins of most leaves, especially near the base. A some- what woody stalk emerges after several years and grows to about 40 ft; lateral branches, themselves usually branched, bear numerous pale yellow flowers along the branches, pendant, 1 inch in diameter, fragrant, 3 inner petals (tepals) positioned be- tween 3 outer ones. Fruits are capsules, cylindrical, contain- ing black seed. Many bulblets (bulbils) capable of developing into new plants are formed on inflorescence. Genus named for French chemist A.F. Fourcroy (d. 1809); foetida for the slightly unpleasant smell of the plant sap(70). Often confused with sisal (Agave sisalana Perrine), also a weed of arid areas. Both were introduced into Hawai‘i in attempts to start a cordage industry(59, 70). The in- florescences of sisal are upright at the end of the branches. Agave sisalana Distribution: Originally from South America, culti- vated for fiber, and thus widely naturalized. Occurs in dry, rocky areas. In Hawai‘i, naturalized on all islands except Niÿihau and Kahoÿolawe. First reported in Management: Tolerant of aqueous sprays of Hawai‘i in 1888(70). glyphosate, hexazinone, and triclopyr and to soil appli- cations of hexazinone. Sensitive to foliar sprays of 2,4- Environmental impact: Displaces other plants in D in diesel and very sensitive to foliar sprays of triclopyr drier forests and pastures. -

Chemical Constituents from the Roots of Furcraea Bedinghausii Koch
International Letters of Chemistry, Physics and Astronomy Online: 2013-08-05 ISSN: 2299-3843, Vol. 16, pp 9-19 doi:10.18052/www.scipress.com/ILCPA.16.9 CC BY 4.0. Published by SciPress Ltd, Switzerland, 2013 Chemical Constituents from the Roots of Furcraea bedinghausii Koch Rémy B. Teponno1,*, Beaudelaire K. Ponou1,2, Dennis Fiorini2, Luciano Barboni2, Léon A. Tapondjou1 1Laboratory of Environmental and Applied Chemistry, Department of Chemistry, Faculty of Science, University of Dschang, PO Box 183, Dschang, Cameroon 2School of Science and Technology, Chemistry Division, University of Camerino, Via S. Agostino 1, I-62032 Camerino, Italy *E-mail address: [email protected] ABSTRACT Phytochemical investigation of the roots of Furcraea bedinghausii Koch. Led to the isolation of a mixture of two new homoisoflavones, 5,7-dihydroxy-3-(3,4-methylenedioxybenzyl)-chromone (4a) and 5,7-dihydroxy-3-(4-methoxybenzyl)-chromone (4b), together with the known β-sitosterol (1), 7,4'-dihydroxyhomoisoflavane (2), dihydrobonducellin (3), kaempferol (5), 5,7-dihydroxy-3-(4- hydroxybenzyl)-chromone (6), 1-linoleylglycerol (7), 6’-linoleyl-3-O-β-D-glucopyranosyl-β-sitosterol (8), trans-3,3’,5,5’-tetrahydroxy-4’-methoxystilbene (9), yuccaol C (10), yuccaol D (11), 3-O-β-D- glucopyranosyl-β-sitosterol (12), 4-[6-O-(4-hydroxy-3,5-dimethoxybenzoyl)-β-D- glucopyranosyloxy]-3-methoxybenzoic acid (13) and two pairs of steroidal saponins: (25R)-2α-3β– dihydroxy-5α-spirostan-12-one 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β- D-glucopyranosyl-(1→4)-β-D-galactopyranoside (14a) and (25R)-2α-3β–dihydroxy-5α-spirost-9-en- 12-one 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl- (1→4)- β-D-galactopyranoside (14b), (25R)-3β–hydroxy-5α-spirostan-12-one 3-O-β-D- glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D- galactopyranoside (15a) and (25R)-3β–hydroxy-5α-spirost-9-en-12-one 3-O-β-D-glucopyranosyl- (1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside (15b). -

Low-Maintenance Landscape Plants for South Florida1
ENH854 Low-Maintenance Landscape Plants for South Florida1 Jody Haynes, John McLaughlin, Laura Vasquez, Adrian Hunsberger2 Introduction regular watering, pruning, or spraying—to remain healthy and to maintain an acceptable aesthetic This publication was developed in response to quality. A low-maintenance plant has low fertilizer requests from participants in the Florida Yards & requirements and few pest and disease problems. In Neighborhoods (FYN) program in Miami-Dade addition, low-maintenance plants suitable for south County for a list of recommended landscape plants Florida must also be adapted to—or at least suitable for south Florida. The resulting list includes tolerate—our poor, alkaline, sand- or limestone-based over 350 low-maintenance plants. The following soils. information is included for each species: common name, scientific name, maximum size, growth rate An additional criterion for the plants on this list (vines only), light preference, salt tolerance, and was that they are not listed as being invasive by the other useful characteristics. Florida Exotic Pest Plant Council (FLEPPC, 2001), or restricted by any federal, state, or local laws Criteria (Burks, 2000). Miami-Dade County does have restrictions for planting certain species within 500 This section will describe the criteria by which feet of native habitats they are known to invade plants were selected. It is important to note, first, that (Miami-Dade County, 2001); caution statements are even the most drought-tolerant plants require provided for these species. watering during the establishment period. Although this period varies among species and site conditions, Both native and non-native species are included some general rules for container-grown plants have herein, with native plants denoted by †. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

UFFLORIDA IFAS Extension
ENH854 UFFLORIDA IFAS Extension Low-Maintenance Landscape Plants for South Florida1 Jody Haynes, John McLaughlin, Laura Vasquez, Adrian Hunsberger2 Introduction The term "low-maintenance" refers to a plant that does not require frequent maintenance-such as This publication was developed in response to regular watering, pruning, or spraying-to remain requests from participants in the Florida Yards & healthy and to maintain an acceptable aesthetic Neighborhoods (FYN) program in Miami-Dade quality. A low-maintenance plant has low fertilizer County for a list of recommended landscape plants requirements and few pest and disease problems. In suitable for south Florida. The resulting list includes addition, low-maintenance plants suitable for south over 350 low-maintenance plants. The following Florida must also be adapted to--or at least information is included for each species: common tolerate-our poor, alkaline, sand- or limestone-based name, scientific name, maximum size, growth rate soils. (vines only), light preference, salt tolerance, and other useful characteristics. An additional criterion for the plants on this list was that they are not listed as being invasive by the Criteria Florida Exotic Pest Plant Council (FLEPPC, 2001), or restricted by any federal, state, or local laws This section will describe the criteria by which (Burks, 2000). Miami-Dade County does have plants were selected. It is important to note, first, that restrictions for planting certain species within 500 even the most drought-tolerant plants require feet of native habitats they are known to invade watering during the establishment period. Although (Miami-Dade County, 2001); caution statements are this period varies among species and site conditions, provided for these species. -
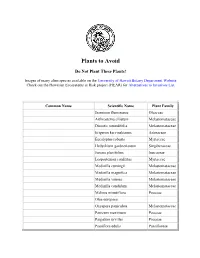
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

A Synopsis of Feral Agave and Furcraea (Agavaceae, Asparagaceae S. Lat.) in the Canary Islands (Spain)
Plant Ecology and Evolution 152 (3): 470–498, 2019 https://doi.org/10.5091/plecevo.2019.1634 REGULAR PAPER A synopsis of feral Agave and Furcraea (Agavaceae, Asparagaceae s. lat.) in the Canary Islands (Spain) Filip Verloove1,*, Joachim Thiede2, Águedo Marrero Rodríguez3, Marcos Salas-Pascual4, Jorge Alfredo Reyes-Betancort5, Elizabeth Ojeda-Land6 & Gideon F. Smith7 1Meise Botanic Garden, Nieuwelaan 38, B-1860 Meise, Belgium 2Schenefelder Holt 3, 22589 Hamburg, Germany 3Jardín Botánico Canario Viera y Clavijo, Unidad Asociada al CSIC, C/ El Palmeral nº 15, Tafira Baja, E-35017 Las Palmas de Gran Canaria, Gran Canaria, Canary Islands, Spain 4Instituto de Estudios Ambientales y Recursos Naturales (i-UNAT), Campus Universitario de Tafira, Universidad de las Palmas de Gran Canaria, E-35017 Las Palmas de Gran Canaria, Gran Canaria, Canary Islands, Spain 5Jardín de Aclimatación de La Orotava (ICIA). C/ Retama 2, 38400 Puerto de la Cruz, Canary Islands, Spain 6Viceconsejería de Medio Ambiente. Gobierno de Canarias. C/ Avda. de Anaga, 35. Planta 11. 38071 Santa Cruz de Tenerife, Canary Islands, Spain 7Department of Botany, P.O. Box 77000, Nelson Mandela University, Port Elizabeth, 6031 South Africa / Centre for Functional Ecology, Departamento de Ciências da Vida, Calçada Martim de Freitas, Universidade de Coimbra, 3001-455 Coimbra, Portugal *Corresponding author: [email protected] Background – Species of Agave and Furcraea (Agavaceae, Asparagaceae s. lat.) are widely cultivated as ornamentals in Mediterranean climates. An increasing number is escaping and naturalising, also in natural habitats in the Canary Islands (Spain). However, a detailed treatment of variously naturalised and invasive species found in the wild in the Canary Islands is not available and, as a result, species identification is often problematic. -

The Naturalized Vascular Plants of Western Australia 1
12 Plant Protection Quarterly Vol.19(1) 2004 Distribution in IBRA Regions Western Australia is divided into 26 The naturalized vascular plants of Western Australia natural regions (Figure 1) that are used for 1: Checklist, environmental weeds and distribution in bioregional planning. Weeds are unevenly distributed in these regions, generally IBRA regions those with the greatest amount of land disturbance and population have the high- Greg Keighery and Vanda Longman, Department of Conservation and Land est number of weeds (Table 4). For exam- Management, WA Wildlife Research Centre, PO Box 51, Wanneroo, Western ple in the tropical Kimberley, VB, which Australia 6946, Australia. contains the Ord irrigation area, the major cropping area, has the greatest number of weeds. However, the ‘weediest regions’ are the Swan Coastal Plain (801) and the Abstract naturalized, but are no longer considered adjacent Jarrah Forest (705) which contain There are 1233 naturalized vascular plant naturalized and those taxa recorded as the capital Perth, several other large towns taxa recorded for Western Australia, com- garden escapes. and most of the intensive horticulture of posed of 12 Ferns, 15 Gymnosperms, 345 A second paper will rank the impor- the State. Monocotyledons and 861 Dicotyledons. tance of environmental weeds in each Most of the desert has low numbers of Of these, 677 taxa (55%) are environmen- IBRA region. weeds, ranging from five recorded for the tal weeds, recorded from natural bush- Gibson Desert to 135 for the Carnarvon land areas. Another 94 taxa are listed as Results (containing the horticultural centre of semi-naturalized garden escapes. Most Total naturalized flora Carnarvon). -

Botanical Identi Cations of Seventeenth Century Plant Illustrations in The
Looking into the ora of Dutch Brazil: botanical identications of seventeenth century plant illustrations in the Libri Picturati Mireia Alcántara-Rodríguez ( [email protected] ) Leiden University Mariana Françozo Leiden University Tinde Van Andel Naturalis Biodiversity Center Research Article Keywords: African heritage, Atlantic Forest, Botanical drawings, Digitalization, Naturalists, Tupi Posted Date: June 14th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-609648/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/31 Abstract The Libri Picturati includes a collection of plant illustrations from seventeenth century Dutch Brazil that is kept in the Jagiellonian library in Krakow since World War II. While many studies focused on the artistic details of these images, we identied the ora depicted. We used contemporary textual sources (e.g., Historia Naturalis Brasiliae), monographs and taxonomist’ assessments. We checked origin, life form, domestication and conservation status and the plant parts that are represented. We identied 198 taxa, consisting mostly of wild, native rainforest trees and 35 introduced species. Fertile branches are the most represented, although some loose dry fruits and sterile material were also painted, which sheds light into the collection methods by naturalists in Dutch Brazil. Several species are no longer abundant or have become invasive due to anthropogenic inuences since colonialism. Through this botanical iconography, we traced the rst records of the sunower and the Ethiopian pepper in Brazil, as well as the dispersion and assimilation of the ora encountered in the colony by Indigenous, African and European peoples. We emphasized the relevance of combining visual and textual sources when studying natural history collections and we highlighted how digitalization makes these artistic and scientic collections more accessible. -

Sapia News Southern African Plant Invaders Atlas
SAPIA NEWS SOUTHERN AFRICAN PLANT INVADERS ATLAS January 2013 ARC-Plant Protection Research Institute No. 27 Invasive plants Stop with You! The problem of invasive alien plants rests with us all —from national government whose responsibility it is to promulgate and enforce laws, and to provide management strategies for implementation by provincial and local government authorities ; to im- porters, growers and sellers of plants who should only deal with non-invasive plants, and ultimately all landowners who are the custodians of the land—whether it be a pocket- sized urban garden or a vast farm or reserve. The problem of alien plant invasion in South Africa is exacerbated by failures to Inside this issue: meet responsibilities at all levels, and that includes government . National govern- ment has failed to implement regulations on invasive species under the National Environ- mental: Biodiversity Act (NEMBA) which was promulgated in 2004. These delays have Invasive plants Stop with You! also delayed the revision of the Conservation of Agricultural Resources Act (CARA). En- High Court action against forcement of laws is, and has always been, inadequate or totally lacking. government Now the people are taking government to court: Early detection of 1 invasive species The Kloof Conservancy in KwaZulu-Natal has initiated High Court proceedings to compel the government to implement Invasive Alien Species legislation. For details, Tribute to Lynne Thompson of ‘Stop-the-Spread’ including copies of the Notice of Motion and the Founding Affidavit, visit the Kloof Conservancy website: www.kloofconservancy.org.za Early Detection: Water poppy 2 & 3 Mauritius hemp Pitch apple—emerging invader 4 Editor and SAPIA co-ordinator: Lesley Henderson ARC-PPRI, Weeds Research Programme c/o SANBI Private Bag X101 Pretoria 0001 Water poppy ( Hydrocleys nymphoides ) South Africa an emerging invasive aquatic plant e-mail: [email protected] Photo: H. -

Jumping the Garden Fence
Jumping the Garden Fence Invasive garden plants in Australia and their environmental and agricultural impacts A CSIRO report for WWF-Australia by R.H. Groves CSIRO Plant Industry Robert Boden Robert Boden & Associates W.M. Lonsdale CSIRO Entomology February 2005 Jumping the Garden Fence: Invasive Garden Plants in Australia © WWF-Australia 2005. All Rights Reserved. ISBN 1 875941 84 3 Authors: Richard Groves, Robert Boden and Mark Lonsdale WWF-Australia Head Office Level 13, 235 Jones St Ultimo NSW 2007 Tel: +612 9281 5515 Fax: +612 9281 1060 www.wwf.org.au Published in February 2005 by WWF-Australia. Any reproduction in full or part of this publication must mention the title and credit the above mentioned publisher as the copyright owner. First published in February 2005 For bibliographic purposes this paper should be cited as: Groves, R.H., Boden, R. & Lonsdale, W.M. 2005. Jumping the Garden Fence: Invasive Garden Plants in Australia and their environmental and agricultural impacts. CSIRO report prepared for WWF-Australia. WWF-Australia, Sydney. The opinions expressed in this publication are those of the authors and do not necessarily reflect the view of WWF. For copies of this report, please contact WWF-Australia at [email protected] or call 1800 032 551. World Wide Fund for Nature ABN: 57 001 594 074 Acknowledgments. We thank Andreas Glanznig for initiating the project and commenting throughout the gestation of this report. Dave Albrecht (Alice Springs), George Batianoff (Qld), Kate Blood (Vic), Geoff Butler and Geoff Price (ACT), David Cooke (SA), John Hosking (NSW), Greg Keighery (WA), Andrew Mitchell (NT Top End) and Tim Rudman (Tas) gave their time and experience to nominate the most important garden plants that were still for sale in their respective jurisdictions. -

Flora of Australia, Volume 46, Iridaceae to Dioscoreaceae
FLORA OF AUSTRALIA Volume 46 Iridaceae to Dioscoreaceae This volume was published before the Commonwealth Government moved to Creative Commons Licensing. © Commonwealth of Australia 1986. This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced or distributed by any process or stored in any retrieval system or data base without prior written permission from the copyright holder. Requests and inquiries concerning reproduction and rights should be addressed to: [email protected] FLORA OF AUSTRALIA The nine families in this volume of the Flora of Australia are Iridaceae, Aloeaceae, Agavaceae, Xanthorrhoeaceae, Hanguan- aceae, Taccaceae, Stemonaceae, Smilacaceae and Dioscoreaceae. The Xanthorrhoeaceae has the largest representation with 10 genera and 99 species. Most are endemic with a few species of Lomandra and Romnalda extending to neighbouring islands. The family includes the spectacular blackboys and grass-trees. The Iridaceae is largely represented by naturalised species with 52 of the 78 species being introduced. Many of the introductions are ornamentals and several have become serious weeds. Patersonia is the largest genus with all 17 species endemic. Some of these are cultivated as ornamentals. The Dioscoreaccae is a family of economic significance, particularly in the old world tropics where some species are cultivated or collected for their tubers and bulbils. In Australia there are 5 species, one of which is a recent introduction. The endemic and native species, commonly known as yams, are traditionally eaten by the Aborigines.