Atomic Oxygen Is of Limited Practical Use Because Most Oxygen Is in the Form of Molecular Oxygen (O2) at Room Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Singlet/Triplet State Anti/Aromaticity of Cyclopentadienylcation: Sensitivity to Substituent Effect
Article Singlet/Triplet State Anti/Aromaticity of CyclopentadienylCation: Sensitivity to Substituent Effect Milovan Stojanovi´c 1, Jovana Aleksi´c 1 and Marija Baranac-Stojanovi´c 2,* 1 Institute of Chemistry, Technology and Metallurgy, Center for Chemistry, University of Belgrade, Njegoševa 12, P.O. Box 173, 11000 Belgrade, Serbia; [email protected] (M.S.); [email protected] (J.A.) 2 Faculty of Chemistry, University of Belgrade, Studentski trg 12-16, P.O. Box 158, 11000 Belgrade, Serbia * Correspondence: [email protected]; Tel.: +381-11-3336741 Abstract: It is well known that singlet state aromaticity is quite insensitive to substituent effects, in the case of monosubstitution. In this work, we use density functional theory (DFT) calculations to examine the sensitivity of triplet state aromaticity to substituent effects. For this purpose, we chose the singlet state antiaromatic cyclopentadienyl cation, antiaromaticity of which reverses to triplet state aromaticity, conforming to Baird’s rule. The extent of (anti)aromaticity was evaluated by using structural (HOMA), magnetic (NICS), energetic (ISE), and electronic (EDDBp) criteria. We find that the extent of triplet state aromaticity of monosubstituted cyclopentadienyl cations is weaker than the singlet state aromaticity of benzene and is, thus, slightly more sensitive to substituent effects. As an addition to the existing literature data, we also discuss substituent effects on singlet state antiaromaticity of cyclopentadienyl cation. Citation: Stojanovi´c,M.; Aleksi´c,J.; Baranac-Stojanovi´c,M. Keywords: antiaromaticity; aromaticity; singlet state; triplet state; cyclopentadienyl cation; substituent Singlet/Triplet State effect Anti/Aromaticity of CyclopentadienylCation: Sensitivity to Substituent Effect. -

Review Sheet on Determining Term Symbols
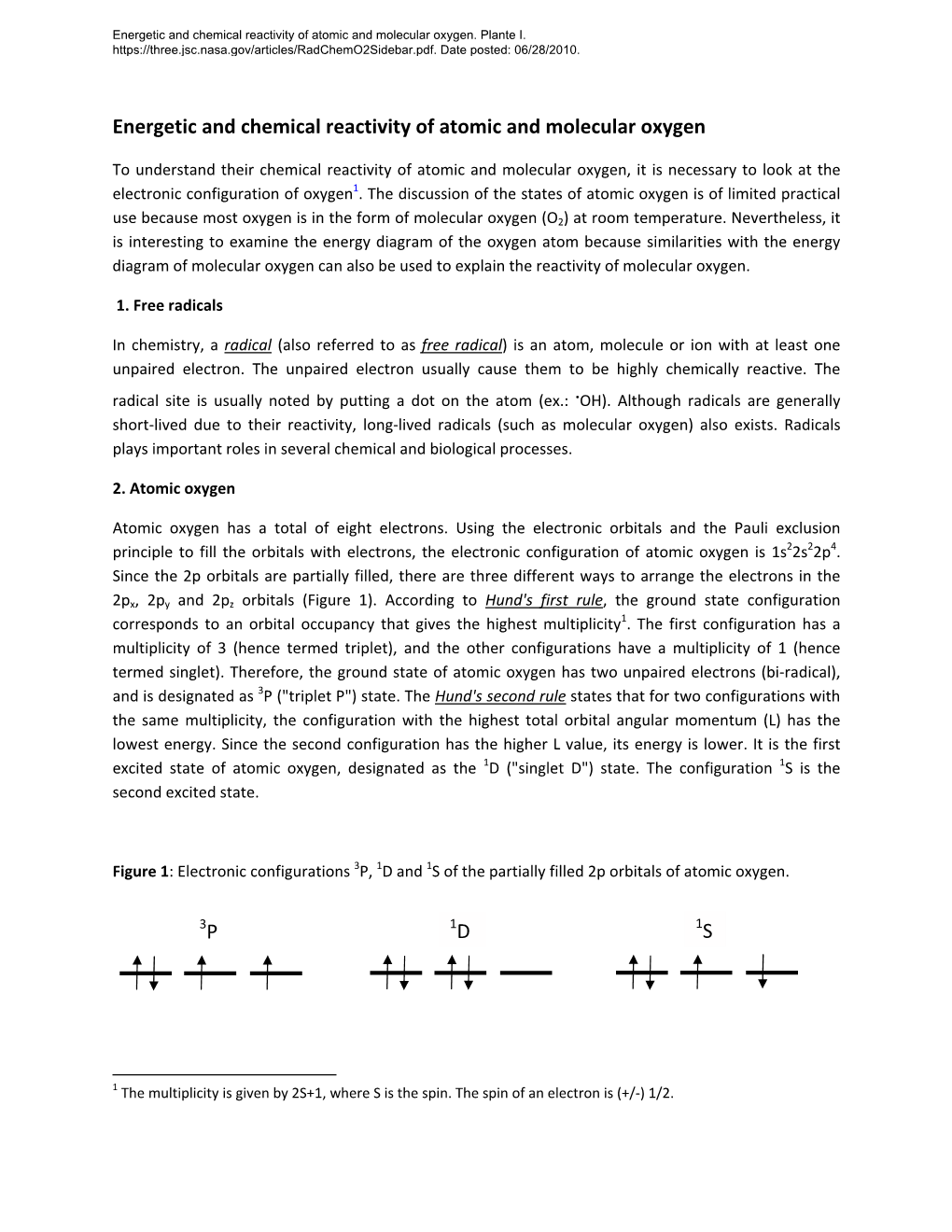
Review Sheet on Determining Term Symbols Term symbols for electronic configurations are useful not only to the spectroscopist but also to the inorganic chemist interested in understanding electronic and magnetic properties of molecules. We will concentrate on the method of Douglas and McDaniel (p. 26ff); however, you may find other treatments equal or superior to the D & M method. Term symbols are a shorthand method used to describe the energy, angular momentum, and spin multiplicity of an atom in any particular state. The general form is a given as Tj where T is a capital letter corresponding to the value of L (the angular momentum quantum number) and may be assigned as S, P, D, F, G, … for |L| = 0, 1, 2, 3, 4, … respectively. The superscript “a” is called the spin multiplicity and can be evaluated as a = 2S +1 where S is the spin quantum number. The subscript “j” is the numerical value of J, a new quantum number defined as: J = l +S, which corresponds to the total orbital and spin angular momentum of the system. The term symbol 3P is read as triplet – Pee state and indicates that there are two unpaired electrons in a state with maximum orbital angular momentum, L=1. The number of microstates (N) of a system corresponds to the total number of distinct arrangements for “e” number of electrons to be placed in “n” number of possible orbital positions. N = # of microstates = n!/(e!(n-e)!) For a set of p orbitals n = 6 since there are 2 positions in each orbital. -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Colour Structures in Supersymmetric QCD
Colour structures in Supersymmetric QCD Jorrit van den Boogaard August 23, 2010 In this thesis we will explain a novel method for calculating the colour struc- tures of Quantum ChromoDynamical processes, or QCD prosesses for short, with the eventual goal to apply such knowledge to Supersymmetric theory. QCD is the theory of the strong interactions, among quarks and gluons. From a Supersymmetric viewpoint, something we will explain in the first chapter, it is interesting to know as much as possible about QCD theory, for it will also tell us something about this Supersymmetric theory. Colour structures in particular, which give the strength of the strong force, can tell us something about the interactions with the Supersymmetric sector. There are a multitude of different ways to calculate these colour structures. The one described here will make use of diagrammatic methods and is therefore somewhat more user friendly. We therefore hope it will see more use in future calculations regarding these processes. We do warn the reader for the level of knowledge required to fully grasp the subject. Although this thesis is written with students with a bachelor degree in mind, one cannot escape touching somewhat dificult matterial. We have tried to explain things as much as possible in a way which should be clear to the reader. Sometimes however we saw it neccesary to skip some of the finer details of the subject. If one wants to learn more about these hiatuses we suggest looking at the material in the bibliography. For convenience we will use some common conventions in this thesis. -

States of Oxygen Liquid and Singlet Oxygen Photodynamic Therapy
Oxygen States of Oxygen As far as allotropes go, oxygen as an element is fairly uninteresting, with ozone (O3, closed-shell and C2v symmetric like SO2) and O2 being the stable molecular forms. Most of our attention today will be devoted to the O2 molecule that is so critically connected with life on Earth. 2 2 2 4 2 O2 has the following valence electron configuration: (1σg) (1σu) (2σg) (1πu) (1πg) . It is be- cause of the presence of only two electrons in the two π* orbitals labelled 1πg that oxygen is paramagnetic with a triplet (the spin multiplicity \triplet" is given by 2S+1; here S, the total spin quantum number, is 0.5 + 0.5 = 1). There are six possible ways to arrange the two electrons in the two degenerate π* orbitals. These different ways of arranging the electrons in the open shell are called \microstates". Further, because there are six microstates, we can say that the total degeneracy of the electronic states 2 3 − arising from (1πg) configuration must be equal to six. The ground state of O2 is labeled Σg , the left superscript 3 indicating that this is a triplet state. It is singly degenerate orbitally and triply degenerate in terms of spin multiplicity; the total degeneracy (three for the ground state) is given by the spin times the orbital degeneracy. 1 The first excited state of O2 is labeled ∆g, and this has a spin degeneracy of one and an orbital degeneracy of two for a total degeneracy of two. This state corresponds to spin pairing of the electrons in the same π* orbital. -

Photochemical and Magnetic Properties of Complex and Nuclear Chemistry
• Programme: MSc in Chemistry • Course Code: MSCHE2001C04 • Course Title: Photochemical and magnetic properties of complex and nuclear chemistry • Unit: Electronic spectra of coordination compounds • Course Coordinator: Dr. Jagannath Roy, Associate Professor, Department of Chemistry, Central University of South Bihar Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data/figures/materials are taken from text books, e-books, research articles, wikipedia and other online resources. 1 Course Objectives: 1. To make students understand structure and propeties of inorganic compounds 2. To accuaint the students with the electronic spectroscopy of coordination compounds 3. To introduce the concepts of magnetochemistry among the students for analysing the properties of complexes. 4. To equip the students with necessary skills in photochemical reaction transition metal complexes 5. To develop knowledge among the students on nuclear and radio chemistry Learning Outcomes: After completion of the course, learners will be able to: 1. Analyze the optical/electronic spectra of coordination compounds 2. Make use of the photochemical behaviour of complexes in designing solar cells and other applications. 3. Design and perform photochemical reactions of transition metal complexes 4. Analyze the magnetic properties of complexes 5. Explain the various phenomena taking place in the nucleus 6. Understand the working of nuclear reactors 2 Lecture-1 Lecture Topic- Introduction to the Electronic Spectra of Transition Metal complexes 3 Crystal field theory (CFT) is ideal for d1 (d9) systems but tends to fail for the more common multi- electron systems. This is because of electron-electron repulsions in addition to the crystal field effects on the repulsion of the metal electrons by the ligand electrons. -

The Chemistry of Carbene-Stabilized
THE CHEMISTRY OF CARBENE-STABILIZED MAIN GROUP DIATOMIC ALLOTROPES by MARIHAM ABRAHAM (Under the Direction of Gregory H. Robinson) ABSTRACT The syntheses and molecular structures of carbene-stabilized arsenic derivatives of 1 1 i 1 1 AsCl3 (L :AsCl3 (1); L : = :C{N(2,6- Pr2C6H3)CH}2), and As2 (L :As–As:L (2)), are presented herein. The potassium graphite reduction of 1 afforded the carbene-stabilized diarsenic complex, 2. Notably, compound 2 is the first Lewis base stabilized diatomic molecule of the Group 13–15 elements, in the formal oxidation state of zero, in the fourth period or lower of the Periodic Table. Compound 2 contains one As–As σ-bond and two lone pairs of electrons on each arsenic atom. In an effort to study the chemistry of the electron-rich compound 2, it was combined with an electron-deficient Lewis acid, GaCl3. The addition of two equivalents of GaCl3 to 2 resulted in one-electron oxidation of 2 to 1 1 •+ – •+ – give [L :As As:L ] [GaCl4] (6 [GaCl4] ). Conversely, the addition of four equivalents of GaCl3 to 2 resulted in two- electron oxidation of 2 to give 1 1 2+ – 2+ – •+ [L :As=As:L ] [GaCl4 ]2 (6 [GaCl4 ]2). Strikingly, 6 represents the first arsenic radical to be structurally characterized in the solid state. The research project also explored the reactivity of carbene-stabilized disilicon, (L1:Si=Si:L1 (7)), with borane. The reaction of 7 with BH3·THF afforded two unique compounds: one containing a parent silylene (:SiH2) unit (8), and another containing a three-membered silylene ring (9). -

Introduction to Molecular Orbital Theory
Chapter 2: Molecular Structure and Bonding Bonding Theories 1. VSEPR Theory 2. Valence Bond theory (with hybridization) 3. Molecular Orbital Theory ( with molecualr orbitals) To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond theory (with hybridization) and Molecular Orbital Theory. A good theory should predict physical and chemical properties of the molecule such as shape, bond energy, bond length, and bond angles.Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. The best it can do is suggest that these molecules are mixtures, or hybrids, of the two Lewis structures that can be written for these molecules. This problem, and many others, can be overcome by using a more sophisticated model of bonding based on molecular orbitals. Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. But this power carries a significant cost in terms of the ease with which the model can be visualized. One model does not describe all the properties of molecular bonds. Each model desribes a set of properties better than the others. The final test for any theory is experimental data. Introduction to Molecular Orbital Theory The Molecular Orbital Theory does a good job of predicting elctronic spectra and paramagnetism, when VSEPR and the V-B Theories don't. -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -

Photochemical Generation of Carbenes and Ketenes from Phenanthrene-Based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene
Colby College Digital Commons @ Colby Honors Theses Student Research 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene Tarini S. Hardikar Student Tarini Hardikar Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Organic Chemistry Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Hardikar, Tarini S. and Hardikar, Tarini, "Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene" (2017). Honors Theses. Paper 948. https://digitalcommons.colby.edu/honorstheses/948 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR A Thesis Presented to the Department of Chemistry, Colby College, Waterville, ME In Partial Fulfillment of the Requirements for Graduation With Honors in Chemistry SUBMITTED MAY 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR Approved: (Mentor: Dasan M. Thamattoor, Professor of Chemistry) (Reader: Rebecca R. Conry, Associate Professor of Chemistry) “NOW WE KNOW” - Dasan M. Thamattoor Vitae Tarini Shekhar Hardikar was born in Vadodara, Gujarat, India in 1996. She graduated from the S.N. -

Covalent Bonding and Molecular Orbitals
Covalent Bonding and Molecular Orbitals Chemistry 35 Fall 2000 From Atoms to Molecules: The Covalent Bond n So, what happens to e- in atomic orbitals when two atoms approach and form a covalent bond? Mathematically: -let’s look at the formation of a hydrogen molecule: -we start with: 1 e-/each in 1s atomic orbitals -we’ll end up with: 2 e- in molecular obital(s) HOW? Make linear combinations of the 1s orbital wavefunctions: ymol = y1s(A) ± y1s(B) Then, solve via the SWE! 2 1 Hydrogen Wavefunctions wavefunctions probability densities 3 Hydrogen Molecular Orbitals anti-bonding bonding 4 2 Hydrogen MO Formation: Internuclear Separation n SWE solved with nuclei at a specific separation distance . How does the energy of the new MO vary with internuclear separation? movie 5 MO Theory: Homonuclear Diatomic Molecules n Let’s look at the s-bonding properties of some homonuclear diatomic molecules: Bond order = ½(bonding e- - anti-bonding e-) For H2: B.O. = 1 - 0 = 1 (single bond) For He2: B.O. = 1 - 1 = 0 (no bond) 6 3 Configurations and Bond Orders: 1st Period Diatomics Species Config. B.O. Energy Length + 1 H2 (s1s) ½ 255 kJ/mol 1.06 Å 2 H2 (s1s) 1 431 kJ/mol 0.74 Å + 2 * 1 He2 (s1s) (s 1s) ½ 251 kJ/mol 1.08 Å 2 * 2 He2 (s1s) (s 1s) 0 ~0 LARGE 7 Combining p-orbitals: s and p MO’s antibonding end-on overlap bonding antibonding side-on overlap bonding antibonding bonding8 4 2nd Period MO Energies s2p has lowest energy due to better overlap (end-on) of 2pz orbitals p2p orbitals are degenerate and at higher energy than the s2p 9 2nd Period MO Energies: Shift! For Z <8: 2s and 2p orbitals can interact enough to change energies of the resulting s2s and s2p MOs. -

Deuterium Isotope Effect in the Radiative Triplet Decay of Heavy Atom Substituted Aromatic Molecules
Deuterium Isotope Effect in the Radiative Triplet Decay of Heavy Atom Substituted Aromatic Molecules J. Friedrich, J. Vogel, W. Windhager, and F. Dörr Institut für Physikalische und Theoretische Chemie der Technischen Universität München, D-8000 München 2, Germany (Z. Naturforsch. 31a, 61-70 [1976] ; received December 6, 1975) We studied the effect of deuteration on the radiative decay of the triplet sublevels of naph- thalene and some halogenated derivatives. We found that the influence of deuteration is much more pronounced in the heavy atom substituted than in the parent hydrocarbons. The strongest change upon deuteration is in the radiative decay of the out-of-plane polarized spin state Tx. These findings are consistently related to a second order Herzberg-Teller (HT) spin-orbit coupling. Though we found only a small influence of deuteration on the total radiative rate in naphthalene, a signi- ficantly larger effect is observed in the rate of the 00-transition of the phosphorescence. This result is discussed in terms of a change of the overlap integral of the vibrational groundstates of Tx and S0 upon deuteration. I. Introduction presence of a halogene tends to destroy the selective spin-orbit coupling of the individual triplet sub- Deuterium (d) substitution has proved to be a levels, which is originally present in the parent powerful tool in the investigation of the decay hydrocarbon. If the interpretation via higher order mechanisms of excited states The change in life- HT-coupling is correct, then a heavy atom must time on deuteration provides information on the have a great influence on the radiative d-isotope electronic relaxation processes in large molecules.