Sukh Dev ARKIVOC 2003 (Iii) 1-7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Result Jmu Kath-Udh-Distt.Pdf
GRAND S.NO. ROLL NO NAME OF CANDIDATE PARENTAGE RESULT TOTAL 1 FW-I/18176 Monika Khajuria Sh. Chaman Lal 173 FAIL 2 FW-I/18177 Neha Kumari Sh. Dharam Chand 229 PASS 3 FW-I/18178 Tanvi Sharma Sh. Vijander Sharam 198 PASS 4 FW-I/18179 Roshni Chanda Sh. Jeet Raj Chanda 237 PASS 5 FW-I/18180 Pooja Devi Sh. Rattan Lal 201 PASS 6 FW-I/18181 Manjeet Kour Sh. Attar Singh 204 PASS 7 FW-I/18182 Daljeet Kour Sh. Mohinder Singh 192 PASS 8 FW-I/18183 Anjali Bhagta Sh. Tirath Ram 243 PASS 9 FW-I/18184 Sunnia Bhatti Sh. David 222 PASS 10 FW-I/18185 Ashwani Devi Sh. Tula Ram 200 PASS 11 FW-I/18186 Prabhjot Kaur Sh. Inderjeet Singh 194 PASS 12 FW-I/18187 Neha Kumari Sh. Sudesh Jamwal 180 FAIL 13 FW-I/18188 Manju Bala Sh. Tirth Ram 195 PASS 14 FW-I/18189 Arti Devi Sh. Sham Lal 198 PASS 15 FW-I/18190 Rekha Devi Sh. Mohinder Lal 213 PASS 1 FW-II/18196 Bindu Kumari Sh. Kartar Chand 187 PASS 2 FW-II/18197 Komal Sh. Rajesh Kumar 197 PASS 3 FW-II/18198 Neha Choudhary Sh. Gurdeep Singh 144 FAIL 4 FW-II/18199 Seema Sharma Sh. Suresh Kumar Sharma 213 PASS 5 FW-II/18200 Waheeda Hamid Tantry Ab. Hamid Tantry 198 PASS 6 FW-II/18201 Tsering Dolkar Sh. Tsering Gyalson 22 ABSENT 7 FW-II/18202 Sangay Dolma Sh. Skarma Stanzin 246 PASS 8 FW-II/18203 Sangeeta Devi Sh. -

Curriculum-Vitae
CURRICULUM-VITAE Name: Y. D. VANKAR Date of Birth: December 5, 1950 Place of Birth: Varanasi, India Academic and Professional Career: Degree/position held Year University/Institution M.Sc. 1971 Banaras Hindu University, Varanasi Ph.D. 1976 National Chemical Laboratory, Pune Research Scientist 1975-76 Hindustan Lever Research Centre, Bombay Post-doctoral Research 1976-77 King’s College, London, U.K. Associate (With Prof. D. I. Davies) -do- 1977-79 University of Southern California, Los, Angeles, U.S.A. (With Prof. G. A. Olah, Nobel Laureate) -do- 1979-80 Rice University, Houston, Texas, U.S.A. (With Prof. E. Wenkert) Lecturer 1981-82 Indian Institute of Technology, Kanpur Assistant Professor 1982-91 Indian Institute of Technology, Kanpur Professor 1991-present Indian Institute of Technology, Kanpur Head of the Department January 2005- Indian Institute of Technology, Kanpur January 2008 S.K. Roy Memorial Chair October 2008- Indian Institute of Technology, Kanpur Professor October 2011 Mr. and Mrs. Giyan Singh Bindra Chair Professor (July 01, 2013- Indian Institute of Technology, Kanpur December 31, 2015) Research Interests: 1. Synthetic Carbohydrate Chemistry of Biological Relevance (Glycosidase Inhibitors) 2. Functionalisation of carbohydrates leading to Glycosyl and Sugar Amino Acids, Amino Sugars and O- and C-Glycosides 3. Development of Newer Synthetic Methods Visiting Appointments: Visiting Scientist Aug. 1985- University of California, San Dec. 1985 Diego, La Jolla, U.S.A. (With Prof. E. Wenkert) Alexander von Humboldt Fellow 1990-91 Universität Konstanz, Konstanz, Germany (With Prof. Dr. R. R. Schmidt) do- May 1995- -do- July 1995 Visiting Scientist Dec. 1996- University of Southern California, Los Angeles, May 1997 U.S.A. -
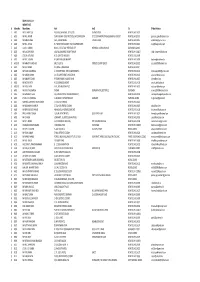
DELHI GOLF CLUB MEMBER LIST Sr Mem.No Mem.Name Add1 Add2 City E-Mail Address 1 A009 MR K
DELHI GOLF CLUB MEMBER LIST Sr Mem.No Mem.Name Add1 Add2 City E-Mail Address 1 A009 MR K. AMRIT LAL 90E, MALCHA MARG, IST FLOOR, CHANKYAPURI NEW DELHI 110021 2 A016 MR N.S. ATWAL GURU MEHAR CONSTRUCTION,S.M.COMPLEX,K-4 G.F.2,OLD RANGPURI ROAD,MAHIPAL PUR EXT. NEW DELHI-110037 [email protected] 3 A018 MR AMAR SINGH 16-A, PALAM MARG VASANT VIHAR, NEW DELHI 110057 [email protected] 4 A021 MR N.S. AHUJA B-7,WEST END COLONY, RAO TULARAM MARG NEW DELHI 110021 [email protected] 5 A022 COL K.C. ANAND BLOCK - 9,FLAT 302 HERITAGE CITY MEHRAULI GURGOAN ROAD GURGOAN 122002 6 A026 MR. ANOOP SINGH 16A, PALAM MARG VASANT VIHAR NEW DELHI- 110057 [email protected] 7 A028 LT GEN. AJIT SINGH R-51, GREATER KAILASH-I, NEW DELHI 110048 8 A032 MR M.T. ADVANI 6-SUNDER NAGAR MARKET, NEW DELHI 110003 [email protected] 9 A035 D1 MR AMARJIT SINGH (II) 680, C-BLOCK, FRIENDS COLONY (EAST) NEW DELHI-110025 [email protected] 10 A042 MR S.S. ANAND N-3,NDSE-1,RING ROAD, NEW DELHI-110049 11 A046 MR VIJAY AGGARWAL 2, CHURCH ROAD, DELHI CANTONMENT, NEW DELHI 110010 [email protected] 12 A051 MR ARJUN ASRANI 12, SFS APARTMENTS HAUZ KHAS NEW DELHI 110016 [email protected] 13 A056 MR AMARJIT SAHAY 9 POORVI MARG VASANT VIHAR NEW DELHI 110057 [email protected] 14 A058 MR ACHAL NATH A 51,NIZAMUDDIN EAST, NEW DELHI 110013 [email protected] 15 A059 D1 MR ATUL NATH A-51, NIZAMUDDIN EAST, NEW DELHI 110013 [email protected] 16 A060 MR. -

Folio Id Name 1 Add 1 Add 2 Add 3 City Pin Net Div War No 1001 Basanth Kumar Subudhi at /Po Binka Bolangir Dist Bolangir Dist Or
UNCLAIMED DIVIDEND FOLIO_ID NAME_1 ADD_1 ADD_2 ADD_3 CITY PIN NET_DIV WAR_NO 1001 BASANTH KUMAR SUBUDHI AT /PO BINKA BOLANGIR DIST BOLANGIR DIST ORRISA 767019 287.50 9115 10012 THRINADHA RAO DUNNA INCOMETAX OFFICE 9-15-10 C B M COMPOUND VISAKHAPATNAM AP 0 287.50 803 10014 THRIPURA BALU N 3/82 AMMAVARISALA STREET RAJAMPET 516 115 CUDDAPAH 516115 575.00 4097 10015 THRIPURAMBA T PARTHA APARTMENTS 12/6 VEMBULI AMMAN KOIL STREET VIRUGAMBAKKAM WEST K K NAGAR MADRAS 600 078 600078 575.00 7304 1002 BASANTI MISHRA D-117 KOEL NAGAR ROURKELA SUNDARGARH ORISSA 769014 575.00 9143 10024 THUMMATHOTI PRAKASAM RETIRED LECTURER IN NURSING NEAR BIG WELL KAPADIPALEM NELLORE 524 001 ANDHRA PRADESH 524001 552.00 4971 10025 TIKAM CHAND JAIN SUREKHA CLOTH MERCHANTS POST DORNAKAL DIST WARANGAL ANDHRA PRADESH 506381 287.50 3796 10029 TIRUPATHY SWAMY GUDALA 5-16-34 SARWABATLA VARI STREET KAVALI-524 201 524201 575.00 5041 10030 TIRUPATI VYANKATRAMANAMDEO JOSHI C/O SHRI N V JOSHI 354 SOMWAR KARAD 415 110 415110 575.00 2455 10035 TIWARI B L NO 83-A/63 TIWARI HOUSE JUHI KANPUR 208 014 U P 208014 1150.00 1217 10038 TONY A NO 44 3RD STREET EAST CLUB ROAD JYOTHI AMMAL NAGAR MADRAS TAMIL NADU 600030 287.50 6938 10042 TRIBHOOVANPAL GOVERDHANDAS S DUGAR BROTHERS & CO. 1116 DALAMAL TOWER 211 NARIMAN POINT BOMBAY 400 021 400021 575.00 1939 10043 TRICHINOPOLY RAMACHANDRAN L NO 6 17TH AVENUE HARRINGTON ROAD MADRAS 600 031 600031 1150.00 6962 10044 TRILOKESWARA RAO TUNUGUNTLA C/O SRI RAGHURAMA TRADERS 2-3-21 UPSTAIRS, GANDHI GUNJ KHAMMAM AP 507003 287.50 3866 10049 TRIUPATHY SWAMY GUDALA 5-16-34 SARVABHATLA VARI STREET KAVALI 524201 524201 287.50 5042 1005 BASAVRAJ ANBANAPPA KORLHALLI 47 ADHARSH NAGAR HUBLI 580032 580032 575.00 5954 10054 TULCHHA DEVI CHORDIYA 64 MULLA SAHIB STREET IST FLOOR SOWCARPET MADRAS 600 079 600079 115.00 7358 10058 TUMMMALA SRI KRISHNA MURTHY "SRINIVASA NILAYAM" FLAT NO.302 3RD FLOOR ADITYA WINDSOR WHITEFIELD KONDAPUR HYDERABAD A.P. -

Department of Social Welfare
District Name Scheme Specific Id Aadhaar Beneficiary Name Address Gender Account BankName IFSCCode Creditted On Credit Amount Credit Status EAST 92669 ********0101 AJIT 215KOTLA VILLAGE F ********0797 DENA BANK - 14-12-2018 2500 Credit Success MAYUR VIHARDELHI91 EAST 73009 ********0064 MAYANK SHARMA 379ANAJ MANDI M ********3125 PUNJAB - 14-12-2018 2500 Credit Success SHAHDARADELHI32 NATIONAL BANK EAST 99162 ********5044 RAM BABU 17102KALYANPURID M ********4033 PUNJAB - 14-12-2018 2500 Credit Success ELHI91 NATIONAL BANK EAST 99163 ********4996 SONU 15125KALYANPURID M ********8573 STATE BANK - 14-12-2018 2500 Credit Success ELHI91 OF INDIA EAST 99161 ********4248 VICKY 126DHOBHI GHAT M ********6753 STATE BANK - 14-12-2018 2500 Credit Success KHICHRIPURDELHI9 OF INDIA 1 EAST 99142 ********1270 NEETU 5229KHICHRIPURDE F ********1670 BANK OF - 14-12-2018 2500 Credit Success LHI91 INDIA EAST 72124 ********6670 HANSIKA SHARMA C9 GNO12 WEST M ********6311 INDIAN - 14-12-2018 2500 Credit Success AZAD NAGAR OVERSEAS KRISHNA NAGAR BANK EAST 75269 ********3101 MOHAMMAD NAASIR KURAISHI 619495TRILOKPURI M ********8023 STATE BANK - 14-12-2018 2500 Credit Success DELHI OF INDIA EAST 75271 ********1887 MOHD ABID A242 BULAND F ********7361 CENTRAL - 14-12-2018 2500 Credit Success MASJIDSHASTRI BANK OF PARKDELHI53 INDIA EAST 100390 ********7548 MEHAR CHAND B293HARIJAN BASTI M ********8763 IDBI BANK - 14-12-2018 2500 Credit Success KONDLIDELHI96 LTD EAST 93457 ********8247 REETA SHARMA 95G NO6OLD M ********6899 BANK OF - 14-12-2018 2500 Credit Success GOVIND -
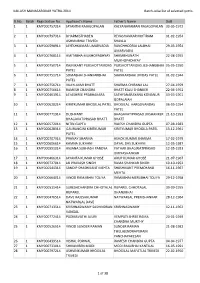
KAILASH MANASAROVAR YATRA-2014 Batch-Wise List of Selected Yatris
KAILASH MANASAROVAR YATRA-2014 Batch-wise list of selected yatris Sl.No. Batch Registration No. -

Year Book of the Indian National Science Academy
AL SCIEN ON C TI E Y A A N C A N D A E I M D Y N E I A R Year Book B of O The Indian National O Science Academy K 2019 2019 Volume I Angkor, Mob: 9910161199 Angkor, Fellows 2019 i The Year Book 2019 Volume–I S NAL CIEN IO CE T A A C N A N D A E I M D Y N I INDIAN NATIONAL SCIENCE ACADEMY New Delhi ii The Year Book 2019 © INDIAN NATIONAL SCIENCE ACADEMY ISSN 0073-6619 E-mail : esoffi [email protected], [email protected] Fax : +91-11-23231095, 23235648 EPABX : +91-11-23221931-23221950 (20 lines) Website : www.insaindia.res.in; www.insa.nic.in (for INSA Journals online) INSA Fellows App: Downloadable from Google Play store Vice-President (Publications/Informatics) Professor Gadadhar Misra, FNA Production Dr VK Arora Shruti Sethi Published by Professor Gadadhar Misra, Vice-President (Publications/Informatics) on behalf of Indian National Science Academy, Bahadur Shah Zafar Marg, New Delhi 110002 and printed at Angkor Publishers (P) Ltd., B-66, Sector 6, NOIDA-201301; Tel: 0120-4112238 (O); 9910161199, 9871456571 (M) Fellows 2019 iii CONTENTS Volume–I Page INTRODUCTION ....... v OBJECTIVES ....... vi CALENDAR ....... vii COUNCIL ....... ix PAST PRESIDENTS OF THE ACADEMY ....... xi RECENT PAST VICE-PRESIDENTS OF THE ACADEMY ....... xii SECRETARIAT ....... xiv THE FELLOWSHIP Fellows – 2019 ....... 1 Foreign Fellows – 2019 ....... 154 Pravasi Fellows – 2019 ....... 172 Fellows Elected (effective 1.1.2019) ....... 173 Foreign Fellows Elected (effective 1.1.2019) ....... 177 Fellowship – Sectional Committeewise ....... 178 Local Chapters and Conveners ...... -

Page 1-68.MDI
C O N T E N T S OVERVIEW i-vi Resource Base vii Performance viii 1.0 S&T CONTRIBUTIONS 1.1 Aerospace Science & Technology 2 1.2 Biology & Biotechnology 6 1.3 Chemical Science & Technology 29 1.4 Earth Resources & Natural Hazards Assessment 44 1.5 Ecology & Environment 50 1.6 Electronics & Instrumentation 57 1.7 Energy 65 1.8 Food & Food Processing 69 1.9 Health Care, Drugs & Pharmaceuticals 79 1.10 Housing & Construction 90 1.11 Information Dissemination & Products 96 1.12 Leather 102 1.13 Material, Minerals, Metals & Manufacturing 105 2.0 CENTRAL MANAGEMENT ACTIVITIES 2.1 CSIR Society 119 2.2 Governing Body 122 2.3 Advisory Board 123 2.4 Department Related Parliamentary Committee 123 2.5 CSIR Foundation Day 124 2.6 Shanti Swarup Bhatnagar Prize 2003: Presentation Ceremony 126 2.7 Shanti Swarup Bhatnagar Prizes 2004 129 2.8 Directors’ Conference 130 3.0 HEADQUARTERS ACTIVITIES 3.1 R&D Planning Division (RDPD) 135 3.2 International S&T Affairs Directorate (ISTAD) 143 3.3 Intellectual Property Management Division (IPMD) 152 3.4 Technology Networking & Business Development Division 154 (TNBD) 3.5 Human Resource Development Group (HRDG) 160 3.6 Human Resource Development Centre (HRDC) 164 3.7 Unit for Science Dissemination (USD) 164 3.8 Recruitment and Assessment Board (RAB) 165 4.0 DATELINE CSIR 169 ANNEXURES I Intellectual Property from CSIR 189 Ia Foreign Patents Granted 190 II Top Papers published by CSIR 213 III Members of CSIR Society (including Members of CSIR 217 Governing Body) IV Members of the Advisory Board 219 V List of Network Projects 220 VI CAG Report 222 VII PM’s Speech at CSIR Society Meeting 224 O V E R V I E W The Annual Report for the year 2004-2005 highlights the salient features of the contributions made by CSIR in a wide spectrum of activities, which span from creation of public goods to private goods to social goods to strategic goods. -

Jammu Province During the Year 2012
Complaints received from Jammu Province during the year 2012 Clt. No. Name of the complainant Name of the suspect Gist of the complaint Disposal at CHQ 01/2012 Ab Rashid Lone S/O Ab Rehman Lone Surinder Kumar Tripathi Managed his appointment in Govt. as JE in Initiated a PV as on 21.1.12 R/O Chanipora Srinagar J&K State pollution control board fraudulently by illegal means 02/2012 Javid Ahmad Dar S/O Ab Gani Dar R/O Lassi Mir Line men Electric Tempered his DOB in his service records. Being anonymous to Kishtawar department Kishtawar records on 24.1.12 03/2012 Kewal Krishan Sharma and others Total Gold Sukh India Ltd company Cheated the applicants fraudulently who have Ref to SSP Distt. Jammu (11) deposited the money in the company as on 21.1.12 as case FIR No 306/11 already registered in P/S Gandhinagar on the same subject. 04/2012 Court direction passed by the Hon’ble Haq Nawaz age 23 years S/O late Forged marriage documents . Case registered on High court Abdul Raheem Mir R/o Zerwa 4.1.2012. Bungwah Tehsil and Distt. Kishtawar 05/2012 Prem Nath Sharma R/O Jawahar Nagar Santosh Kumari D/O Late Amar Managed fake DOB in service records . Being addressed to the Rajouri J&K State Nath W/O Krishan Lal recently Rtd concerned authority to as peon GGHS Murad Pore Rajouri records as on 24.1.12 06/2012 Anrik Singh H.No 162 sector 7 Street 6 Gold Sukh Trade India Ltd Cheating the complainant fraudulently Ref to SSP Distt. -

The Year Book 2019
THE YEAR BOOK 2019 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-2361 6094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2019 Indian Academy of Sciences Information in this Year Book is updated up to 22 February 2019. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Venugopal, M.S. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by Lotus Printers Pvt. Ltd., Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Memorandum of Association ................................................... 2 Role of the Academy ............................................................... 4 Statutes .................................................................................. 7 Council for the period 2019–2021 ............................................ 18 Office Bearers ......................................................................... 19 Former Presidents ................................................................... 20 Activities – a profile ................................................................. 21 Academy Document on Scientific Values ................................. 25 The Academy Trust ................................................................. 33 Section B: Professorships -

General Kofi A. Annan the United Nations United Nations Plaza
MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF PHYSICS CAMBRIDGE, MASSACHUSETTS O2 1 39 October 10, 1997 HENRY W. KENDALL ROOM 2.4-51 4 (617) 253-7584 JULIUS A. STRATTON PROFESSOR OF PHYSICS Secretary- General Kofi A. Annan The United Nations United Nations Plaza . ..\ U New York City NY Dear Mr. Secretary-General: I have received your letter of October 1 , which you sent to me and my fellow Nobel laureates, inquiring whetHeTrwould, from time to time, provide advice and ideas so as to aid your organization in becoming more effective and responsive in its global tasks. I am grateful to be asked to support you and the United Nations for the contributions you can make to resolving the problems that now face the world are great ones. I would be pleased to help in whatever ways that I can. ~~ I have been involved in many of the issues that you deal with for many years, both as Chairman of the Union of Concerne., Scientists and, more recently, as an advisor to the World Bank. On several occasions I have participated in or initiated activities that brought together numbers of Nobel laureates to lend their voices in support of important international changes. -* . I include several examples of such activities: copies of documents, stemming from the . r work, that set out our views. I initiated the World Bank and the Union of Concerned Scientists' examples but responded to President Clinton's Round Table initiative. Again, my appreciation for your request;' I look forward to opportunities to contribute usefully. Sincerely yours ; Henry; W. -

Year Book 2016
INDIAN ACADEMY OF SCIENCES YEAR BOOK 2016 Postal Address Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post Bengaluru 560 080 India Telephone : (080) 2266 1200, 2266 1203 Fax : (080) 2361 6094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2016 Indian Academy of Sciences Text formatted by Wintecs Typesetters, Bengaluru (Ph. 2332 7311) Printed by Brilliant Printers Pvt Ltd., Bengaluru (Ph. 2341 2455) CONTENTS Page Memorandum of Association. 1 Role of the Academy. 3 Statutes. 6 Council for the period 2016–2018. 17 Office Bearers. 18 Former Presidents. 19 Raman Chair. 20 Jubilee Professor. 23 The Fellowship Fellows. 25 Abbreviations. 205 Honorary Fellows. 206 Fellows and Honorary Fellow elected in 2015. 214 Subject-wise list of Fellows. 215 Fellows deceased in 2015. 253 Fellows deceased since 1934. 254 Honorary Fellows deceased since 1934. 270 Associates Associates on roll. 272 Associates selected in 2015. 279 Former Associates. .279 Publications . .289 Committees Sectional Committees. 296 Raman Chair Sub-Committee. 297 Jubilee Professor Sub-Committee . 297 Publications Committee. 298 Joint Science Education Panel . 298 Panel on Scientific Values. 299 Panel on Women in Science. 299 Investment Committee. .299 Standing Committee on Staff. 299 Venues of Annual Meetings. .300 Office Staff. .301 Calendar of Meetings in 2016. 304 List of Holidays in 2016. 304 1 MEMORANDUM OF ASSOCIATION* I. The name of the Association shall be ‘The Indian Academy of Sciences’. II. The objects of the Academy are: 1. To promote the progress and uphold the cause of science, both in pure and applied branches.