Page 1-68.MDI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Part 05.Indd
PART MISCELLANEOUS 5 TOPICS Awards and Honours Y NATIONAL AWARDS NATIONAL COMMUNAL Mohd. Hanif Khan Shastri and the HARMONY AWARDS 2009 Center for Human Rights and Social (announced in January 2010) Welfare, Rajasthan MOORTI DEVI AWARD Union law Minister Verrappa Moily KOYA NATIONAL JOURNALISM A G Noorani and NDTV Group AWARD 2009 Editor Barkha Dutt. LAL BAHADUR SHASTRI Sunil Mittal AWARD 2009 KALINGA PRIZE (UNESCO’S) Renowned scientist Yash Pal jointly with Prof Trinh Xuan Thuan of Vietnam RAJIV GANDHI NATIONAL GAIL (India) for the large scale QUALITY AWARD manufacturing industries category OLOF PLAME PRIZE 2009 Carsten Jensen NAYUDAMMA AWARD 2009 V. K. Saraswat MALCOLM ADISESHIAH Dr C.P. Chandrasekhar of Centre AWARD 2009 for Economic Studies and Planning, School of Social Sciences, Jawaharlal Nehru University, New Delhi. INDU SHARMA KATHA SAMMAN Mr Mohan Rana and Mr Bhagwan AWARD 2009 Dass Morwal PHALKE RATAN AWARD 2009 Actor Manoj Kumar SHANTI SWARUP BHATNAGAR Charusita Chakravarti – IIT Delhi, AWARDS 2008-2009 Santosh G. Honavar – L.V. Prasad Eye Institute; S.K. Satheesh –Indian Institute of Science; Amitabh Joshi and Bhaskar Shah – Biological Science; Giridhar Madras and Jayant Ramaswamy Harsita – Eengineering Science; R. Gopakumar and A. Dhar- Physical Science; Narayanswamy Jayraman – Chemical Science, and Verapally Suresh – Mathematical Science. NATIONAL MINORITY RIGHTS MM Tirmizi, advocate – Gujarat AWARD 2009 High Court 55th Filmfare Awards Best Actor (Male) Amitabh Bachchan–Paa; (Female) Vidya Balan–Paa Best Film 3 Idiots; Best Director Rajkumar Hirani–3 Idiots; Best Story Abhijat Joshi, Rajkumar Hirani–3 Idiots Best Actor in a Supporting Role (Male) Boman Irani–3 Idiots; (Female) Kalki Koechlin–Dev D Best Screenplay Rajkumar Hirani, Vidhu Vinod Chopra, Abhijat Joshi–3 Idiots; Best Choreography Bosco-Caesar–Chor Bazaari Love Aaj Kal Best Dialogue Rajkumar Hirani, Vidhu Vinod Chopra–3 idiots Best Cinematography Rajeev Rai–Dev D Life- time Achievement Award Shashi Kapoor–Khayyam R D Burman Music Award Amit Tivedi. -

Phosphoinositide Signalling in Cell Biology
Photography by Jeswin Singh, Research Scholar, Sumantra Chattarji lab NOTE FROM THE DIRECTOR 9 MAP OF RESEARCH INTERESTS 10 RESEARCH REPORTS Biochemistry, Biophysics and Bioinformatics 12 Cellular Organization and Signalling 20 Neurobiology 28 Genetics and Development 36 Theory, Simulation and Modeling of Biological Systems 44 Ecology and Evolution 52 NEW FACULTY 62 MEETINGS AND WORKSHOPS 68 ACADEMICS AND ADMINISTRATION 70 Academic programmes at NCBS 72 Administration and Finance 74 Research Development Office 76 Research Facilities 78 HIGHLIGHTS OF THE YEAR 82 Twenty-fifth anniversary celebrations 84 Annual talks and Alumni Meet 86 The NCBS Museum and Field Station 88 CONTENTS The NCBS Pachmarhi field station 89 Chemical Ecology network programme 90 The Archives at NCBS 91 Professor Mitradas M Panicker Retires 92 NCBS INTERNATIONAL COLLABORATIONS 94 NCBS NATIONAL COLLABORATIONS 96 A 1993 sketch by Obaid Siddiqi, Founder, National Centre for Biological Sciences, of possible areas to grow at NCBS NCBS at crossroads: 2017 The celebrations of our 25th year have come to an end, and as we reflect back at all that was showcased about our scientific efforts in various meetings and workshops, it is apparent that the vision of NCBS as articulated by Obaid Siddiqi (see his drawing -Biology across scales) is being realized in full measure. This is indeed a terrific achievement and we would be justified in saying that we have laid a strong foundation for a unique scientific institution. However, I strongly feel that we are at a fork in our journey ahead. A number of events over the past years necessitate a reevaluation of the functioning of NCBS for our sustained growth and relevance, and to help us chart a path for our future trajectory. -

Visit for More Placement Papers
Visit www.downloadmela.com for more placement papers FCI tips and trick to crack aptitude questions,Aptitude questions tips and tricks ,Important formulaes,FCI general awareness general anility questions with answers for practice,FCI model question and answers for practice,FCI free solved sample placement papers Finding number of Factors To find the number of factors of a given number, express the number as a product of powers of prime numbers. In this case, 48 can be written as 16 * 3 = (24 * 3) Now, increment the power of each of the prime numbers by 1 and multiply the result. In this case it will be (4 + 1)*(1 + 1) = 5 * 2 = 10 (the power of 2 is 4 and the power of 3 is 1) Therefore, there will 10 factors including 1 and 48. Excluding, these two numbers, you will have 10 – 2 = 8 factors. Sum of n natural numbers -> The sum of first n natural numbers = n (n+1)/2 -> The sum of squares of first n natural numbers is n (n+1)(2n+1)/6 -> The sum of first n even numbers= n (n+1) -> The sum of first n odd numbers= n^2 Finding Squares of numbers To find the squares of numbers near numbers of which squares are known To find 41^2 , Add 40+41 to 1600 =1681 To find 59^2 , Subtract 60^2-(60+59) =3481 Finding number of Positive Roots If an equation (i:e f(x)=0 ) contains all positive co-efficient of any powers of x , it has no positive roots then. -

Result Jmu Kath-Udh-Distt.Pdf
GRAND S.NO. ROLL NO NAME OF CANDIDATE PARENTAGE RESULT TOTAL 1 FW-I/18176 Monika Khajuria Sh. Chaman Lal 173 FAIL 2 FW-I/18177 Neha Kumari Sh. Dharam Chand 229 PASS 3 FW-I/18178 Tanvi Sharma Sh. Vijander Sharam 198 PASS 4 FW-I/18179 Roshni Chanda Sh. Jeet Raj Chanda 237 PASS 5 FW-I/18180 Pooja Devi Sh. Rattan Lal 201 PASS 6 FW-I/18181 Manjeet Kour Sh. Attar Singh 204 PASS 7 FW-I/18182 Daljeet Kour Sh. Mohinder Singh 192 PASS 8 FW-I/18183 Anjali Bhagta Sh. Tirath Ram 243 PASS 9 FW-I/18184 Sunnia Bhatti Sh. David 222 PASS 10 FW-I/18185 Ashwani Devi Sh. Tula Ram 200 PASS 11 FW-I/18186 Prabhjot Kaur Sh. Inderjeet Singh 194 PASS 12 FW-I/18187 Neha Kumari Sh. Sudesh Jamwal 180 FAIL 13 FW-I/18188 Manju Bala Sh. Tirth Ram 195 PASS 14 FW-I/18189 Arti Devi Sh. Sham Lal 198 PASS 15 FW-I/18190 Rekha Devi Sh. Mohinder Lal 213 PASS 1 FW-II/18196 Bindu Kumari Sh. Kartar Chand 187 PASS 2 FW-II/18197 Komal Sh. Rajesh Kumar 197 PASS 3 FW-II/18198 Neha Choudhary Sh. Gurdeep Singh 144 FAIL 4 FW-II/18199 Seema Sharma Sh. Suresh Kumar Sharma 213 PASS 5 FW-II/18200 Waheeda Hamid Tantry Ab. Hamid Tantry 198 PASS 6 FW-II/18201 Tsering Dolkar Sh. Tsering Gyalson 22 ABSENT 7 FW-II/18202 Sangay Dolma Sh. Skarma Stanzin 246 PASS 8 FW-II/18203 Sangeeta Devi Sh. -

Academy News
Proc Indian Natn Sci Acad 85 No. 4 December 2019 pp. 1067-1090 Printed in India. ACADEMY NEWS INSA MEETINGS to Professor Tarun Kant, FNA, Professor Emeritus, Department of Civil Engineering, Several meetings were held during April 08-10, 2019 Indian Institute of Technology Bombay, Powai, in the Academy premises. Mumbai. These included meetings of the different Sectional 4. Professor K Naha Memorial Medal to Committees for recommending names of Young Professor SS Rai, FNA, Professor Emeritus, Scientist Awardees and for the first round of short- Department of Earth and Climate Science, listing of nominations for INSA Fellowship. The Indian Institute of Science Education & Advisory Boards for the various INSA Awards also Research (IISER), Pune. met. These were followed by meetings of the Council and General Body. (C) Endowment Lectures INSA Medal/Lecture Awards 2019 5. Professor Darshan Ranganathan Memorial Lecture to Professor Gaiti Hasan, FNA, The Academy at its General Body Meeting on April National Centre for Biological Sciences, Tata 10, 2019 announced the following six medal/lecture Institute of Fundamental Research, Bengaluru. Awards for 2019. In addition, a new endowment award named International Award Professor TV Desikachary Memorial Medal was also 1. PMS Blackett Memorial Lecture to Sir Tom instituted. The Medal will be awarded to an eminent L Blundell, FNA, Emeritus Professor and scientist for his outstanding contributions in any area Director of Research, Department of of Biological Sciences. The award carries an Biochemistry, University of Cambridge, honorarium of Rs. 25,000/, a bronze medal and a Cambridge. citation. The first medal will be awarded in 2020. -

Sukh Dev ARKIVOC 2003 (Iii) 1-7
Issue in Honor of Prof. Sukh Dev ARKIVOC 2003 (iii) 1-7 Professor Sukh Dev A Tribute Sukh Dev was born to Hari Chand Lala and Maya Vanti at Chakwal (undivided India, now in Pakistan) in June 1923. He started his career in chemistry in 1944 and graduated at D.A.V. College, Lahore (M.Sc. 1945), and later carried out research at Indian Institute of Science, Bangalore (Ph.D., 1950 and D.Sc. 1960). Professor Sukh Dev has been engaged in organic chemistry research for more than half-a-century, and has moved through the laboratories of Indian Institute of Science, Bangalore (1945-1959), to the National Chemical Laboratory, Pune (1960-1974), to the Malti- Chem Research Centre, Nandesari (1974-1988), to Indian Institute of Technology, New Delhi (1989-1993) and at present in Delhi University as a visiting professor in Dr. B. R. Ambedkar centre for biomedical research. As he moved from one institution to another, the thrust of his research effort underwent shifts in consonance with spirit of the institution, ranging from curiosity-oriented investigations to market driven industry/technology oriented research. Professor Sukh Dev's research interest covered a broad range from natural products chemistry, non-benzenoid hydrocarbons, organic reactions, reagents and techniques, drug development from Ayurvedic leads, to technology development. All this activity has led to significant contributions (>375 publications including 50 patents, 92 Ph.D. dissertations), which have been well recognized both nationally and internationally (received a large number of awards and became a fellow of several science academies). He served as a member of the Editorial Boards of Tetrahedron and Tetrahedron Letters (1976-1995), Tetrahedron Asymmetry (1990-1995) and Dictionary of Organic Compounds (5th edition). -

Current Affairs Questions and Answers Pdf – March 2018
CURRENT AFFAIRS QUESTIONS AND ANSWERS PDF – MARCH 2018 1. Which cards Indian Railways has announced that no transaction fees on booking rail tickets? a) Credit card b) Debit card c) Aadhar card d) Ration card Answer b) Debit card. The Indian Railways has announced that Merchant Discount Rate (MDR) charges won’t be levied on passengers for booking railway tickets through debit cards. 2. Which government ties up with Wikipedia to promote their language? a) Tamilnadu b) Kerala c) Karnataka d) Maharastra Answer d) Maharastra. In a first, the Maharashtra government has announced a collaboration with online encyclopedia Wikipedia to promote Marathi globally and increase its online usage. 3. Which company CEO has ranked ahead of Microsoft Co-founder Bill Gates on Hurun Global Rich List 2018? a) JPMorgan Chase b) GEICO c) Berkshie Hathaway d) Coldwell Banker Answer c) Berkshie Hathaway. Berkshire Hathaway CEO Warren Buffett has ranked ahead of Microsoft Co-founder Bill Gates on Hurun Global Rich List 2018. Buffett retained the second spot with a 31% increase in his wealth and became the second person ever to break through the $100-billion barrier. 4. India ranks ______ on Hurun rich list with 131 billionaires? a) Third b) Second c) Fourth d) Fifth Answer a) third. India replaced Germany to reclaim the third spot on the Hurun Global Rich List 2018 with 131 billionaires. India added 31 new billionaires over the last year while the combined wealth of the Indian billionaires increased by 49% to $454 billion. 5. How many Language does Amazon music streaming supports? a) 10 b) 11 c) 14 d) 12 Answer d) 12. -

SUPER Current Affairs MCQ PDF 27Th March 2021 by Dream Big Institution Team (SUPER Current Affairs) © Q
SUPER Current Affairs MCQ PDF 27th March 2021 By Dream Big Institution Team (SUPER Current Affairs) © Q. General Bipin Rawat has formally commissioned the ICGS Vajra into service in Chennai. The Indian Coast Guard Ship VAJRA is _______________ in the series of 7 OPVs being built by L&T A) 5th C) 7th B) 6th D) 4th Answer: B The Chief of Defence staff General Bipin Rawat formally commissioned into service, the Indian Coast Guard ship ‘Vajra’, at the Chennai Port Trust on 24 March 2021. ICGS Vajra is sixth in the series of seven Offshore Patrol Vessel constructed by M/s Larsen & Toubro Ltd. About ICGS Samrat: Length: 105 m Draft: 3.6 m Beam: 13 m Builder: Goa Shipyard Sensors and processing systems: Raytheon surface search radar Displacement: 2,230 short tons (2,020 t) About Bipin Rawat: Born: 16 March 1958 (age 63 years), Pauri Garhwal Spouse: Madhulika Rawat Parents: Lachu Singh Rawat Office: Chief of Defence Staff since 2019 Education: University of Madras, Defence Services Staff College Awards: Param Vishisht Seva Medal, Ati Vishisht Seva Medal About Larsen & Toubro Ltd: CEO: S. N. Subrahmanyan (Jul 2017–) Founded: 7 February 1938, Mumbai Page 1 Follow us: Official Site, Telegram, Facebook, Instagram, Instamojo Headquarters: Mumbai Subsidiaries: LTI, L&T Technology Services, Mindtree Founders: Henning Holck-Larsen, Søren Kristian Toubro Q. Who has been appointed as the CEO of the Unique Identification Authority of India (UIDAI)? A) Saurbah Garg C) Madanlal Rathore B) S.P. Saravanan D) Faiz Ahmed Kidwai Answer: A About -

ANNUAL REPORT 2019-20 IIT Bombay Annual Report 2019-20 Content
IIT BOMBAY ANNUAL REPORT 2019-20 IIT BOMBAY ANNUAL REPORT 2019-20 Content 1) Director’s Report 05 2) Academic Programmes 07 3) Research and Development Activities 09 4) Outreach Programmes 26 5) Faculty Achievements and Recognitions 27 6) Student Activities 31 7) Placement 55 8) Society For Innovation And Entrepreneurship 69 9) IIT Bombay Research Park Foundation 71 10) International Relations 73 11) Alumni And Corporate Relations 84 12) Institute Events 90 13) Facilities 99 a) Infrastructure Development b) Central Library c) Computer Centre d) Centre For Distance Engineering Education Programme 14) Departments/ Centres/ Schools and Interdisciplinary Groups 107 15) Publications 140 16) Organization 141 17) Summary of Accounts 152 Director's Report By Prof. Subhasis Chaudhuri, Director, IIT Bombay Indian Institute of Technology Bombay acknowledged for their research contributions. (IIT Bombay) has a rich tradition of pursuing We have also been able to further our links with excellence and has continually re-invented international and national peer universities, itself in terms of academic programmes and enabling us to enhance research and educational research infrastructure. Students are exposed programmes at the Institute. to challenging, research-based academics and IIT Bombay continues to make forays into a host of sport, cultural and organizational newer territories pertinent to undergraduate activities on its vibrant campus. The presence and postgraduate education. At postgraduate of world-class research facilities, vigorous level, a specially designed MA+PhD dual institute-industry collaborations, international degree programme in Philosophy under the exchange programmes, interdisciplinary HSS department has been introduced. IDC, the research collaborations and industrial training Industrial Design Centre, celebrated 50 years opportunities help the students of IIT Bombay to of its golden existence earlier this year. -

Curriculum-Vitae
CURRICULUM-VITAE Name: Y. D. VANKAR Date of Birth: December 5, 1950 Place of Birth: Varanasi, India Academic and Professional Career: Degree/position held Year University/Institution M.Sc. 1971 Banaras Hindu University, Varanasi Ph.D. 1976 National Chemical Laboratory, Pune Research Scientist 1975-76 Hindustan Lever Research Centre, Bombay Post-doctoral Research 1976-77 King’s College, London, U.K. Associate (With Prof. D. I. Davies) -do- 1977-79 University of Southern California, Los, Angeles, U.S.A. (With Prof. G. A. Olah, Nobel Laureate) -do- 1979-80 Rice University, Houston, Texas, U.S.A. (With Prof. E. Wenkert) Lecturer 1981-82 Indian Institute of Technology, Kanpur Assistant Professor 1982-91 Indian Institute of Technology, Kanpur Professor 1991-present Indian Institute of Technology, Kanpur Head of the Department January 2005- Indian Institute of Technology, Kanpur January 2008 S.K. Roy Memorial Chair October 2008- Indian Institute of Technology, Kanpur Professor October 2011 Mr. and Mrs. Giyan Singh Bindra Chair Professor (July 01, 2013- Indian Institute of Technology, Kanpur December 31, 2015) Research Interests: 1. Synthetic Carbohydrate Chemistry of Biological Relevance (Glycosidase Inhibitors) 2. Functionalisation of carbohydrates leading to Glycosyl and Sugar Amino Acids, Amino Sugars and O- and C-Glycosides 3. Development of Newer Synthetic Methods Visiting Appointments: Visiting Scientist Aug. 1985- University of California, San Dec. 1985 Diego, La Jolla, U.S.A. (With Prof. E. Wenkert) Alexander von Humboldt Fellow 1990-91 Universität Konstanz, Konstanz, Germany (With Prof. Dr. R. R. Schmidt) do- May 1995- -do- July 1995 Visiting Scientist Dec. 1996- University of Southern California, Los Angeles, May 1997 U.S.A. -
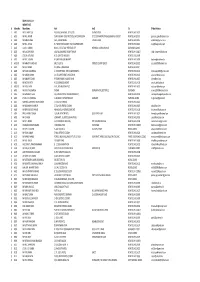
DELHI GOLF CLUB MEMBER LIST Sr Mem.No Mem.Name Add1 Add2 City E-Mail Address 1 A009 MR K
DELHI GOLF CLUB MEMBER LIST Sr Mem.No Mem.Name Add1 Add2 City E-Mail Address 1 A009 MR K. AMRIT LAL 90E, MALCHA MARG, IST FLOOR, CHANKYAPURI NEW DELHI 110021 2 A016 MR N.S. ATWAL GURU MEHAR CONSTRUCTION,S.M.COMPLEX,K-4 G.F.2,OLD RANGPURI ROAD,MAHIPAL PUR EXT. NEW DELHI-110037 [email protected] 3 A018 MR AMAR SINGH 16-A, PALAM MARG VASANT VIHAR, NEW DELHI 110057 [email protected] 4 A021 MR N.S. AHUJA B-7,WEST END COLONY, RAO TULARAM MARG NEW DELHI 110021 [email protected] 5 A022 COL K.C. ANAND BLOCK - 9,FLAT 302 HERITAGE CITY MEHRAULI GURGOAN ROAD GURGOAN 122002 6 A026 MR. ANOOP SINGH 16A, PALAM MARG VASANT VIHAR NEW DELHI- 110057 [email protected] 7 A028 LT GEN. AJIT SINGH R-51, GREATER KAILASH-I, NEW DELHI 110048 8 A032 MR M.T. ADVANI 6-SUNDER NAGAR MARKET, NEW DELHI 110003 [email protected] 9 A035 D1 MR AMARJIT SINGH (II) 680, C-BLOCK, FRIENDS COLONY (EAST) NEW DELHI-110025 [email protected] 10 A042 MR S.S. ANAND N-3,NDSE-1,RING ROAD, NEW DELHI-110049 11 A046 MR VIJAY AGGARWAL 2, CHURCH ROAD, DELHI CANTONMENT, NEW DELHI 110010 [email protected] 12 A051 MR ARJUN ASRANI 12, SFS APARTMENTS HAUZ KHAS NEW DELHI 110016 [email protected] 13 A056 MR AMARJIT SAHAY 9 POORVI MARG VASANT VIHAR NEW DELHI 110057 [email protected] 14 A058 MR ACHAL NATH A 51,NIZAMUDDIN EAST, NEW DELHI 110013 [email protected] 15 A059 D1 MR ATUL NATH A-51, NIZAMUDDIN EAST, NEW DELHI 110013 [email protected] 16 A060 MR. -

Mr. Gautam Rajadhyaksha's Careerscape and Contribution
Mr. Gautam Rajadhyaksha’s Careerscape and Contribution Compiled by Mangesh Chavarkar Gautam Rajadhyaksha is India’s best-known portrait photographer whose definitive portraits have acquired an iconic standard both in India and abroad. ADVERTISING: Mumbai born and educated, Gautam taught Chemistry at his Alma Mater, the St. Xavier’s College, Mumbai, between 1971 and 1973. After Winning a Gold Medal at the College of Advertising and Public Relations, Gautam joined the famous advertising agency Lintas India Limited where he became, primarily, the Head of the Photo Services Department and then later as the Creative Director. During his tenure in Lintas, he participated in the creation and production of such landmark advertising campaigns such as: 1. Liril 2. Fair ‘n Lovely 3. Cherry Blossom 4. Rexona 5. Rin 6. Surf-the ‘Lalitaji’ Campaign Apart from the commercial products that he helped launch, he was a part of Mr. Alyque Padamsee’s Public Awareness campaigns for: 1. Save The Tiger (for the World Wild Life) 1973 2. The ‘Sukhdi’ Food Programme (for the draught relief in 1973-74). From 1985-87, Gautam was solely responsible for the conceptualizing, creation and production of advertising and promotional material for 14 export-oriented products for The Government of India’s Trade Development Authority. During his tenure in advertising, his contribution to the fledgling world of professional photography and modelling has been significant: 1. Grading and standardizing rates of professional photographers. 2. Procedures set to pay photographers within 60 days of shooting. 3. The system of payment of 50% advance on out-of station assignments 4.