Aluminium Hydrides and Borohydrides As Reducing Agents
Total Page:16
File Type:pdf, Size:1020Kb
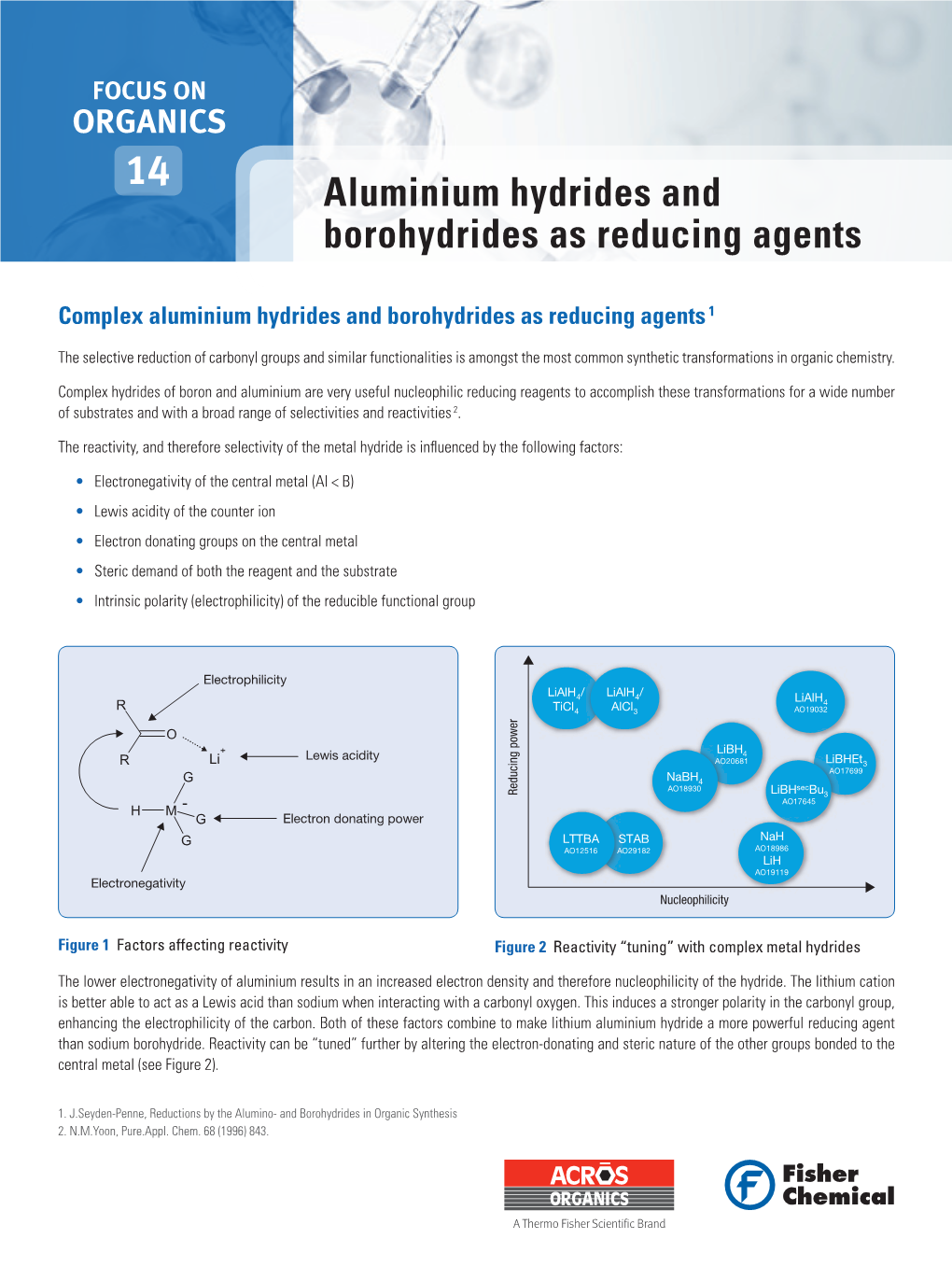
Load more
Recommended publications
-

United States Patent 19 11 Patent Number: 5,418,243 Angerbauer Et Al
USOO5418243A United States Patent 19 11 Patent Number: 5,418,243 Angerbauer et al. 45 Date of Patent: May 23, 1995 54 SUBSTITUTED 4-PHENYL-PYRIDONES 4,215,126 7/1980. Durant et al........................ 514/345 AND 4-PHENYL-3-ALKOXYPYRIDNES 4,684,477 8/1987 Sugimori et al. ... 546/290 4,916,239 4/1990 Treiber ................. ... 549/292 75 Inventors: Rolf Angerbauer; Peter Fey; Walter 4,988,711 1/1991 Angerbauer et al. ... 514/326 Hibsch, all of Wuppertal; Thomas 5,032,602 7/1991 Fey et al. ................. ... 54/345 Philipps, Cologne; Hilmar Bischoff, 5,064,841 11/1991 Angerbauer et al. ... 514/336 Wuppertal; Hans-Peter Krause, 5,138,090 8/1992 Fey et al. .............................. 560/59 Schwelm; Jörg Peterson-von Gehr, Bochum; Delf Schmidt, Wuppertal, FOREIGN PATENT DOCUMENTS all of Germany 0373423 6/1990 European Pat. Off. 73 Assignee: Bayer Aktiengesellschaft, OTHER PUBLICATIONS Leverkusen, Germany J. Med. Chem., 1990, vol. 33, pp. 52-60; “Synthesis and 21) Appl. No.: 166,775 Biological Activity of New HGM-CoA Reductase 22 Filed: Dec. 14, 1993 Inhibitors ... ', G. Beck. Primary Examiner-C. Warren Ivy (30) Foreign Application Priority Data Assistant Examiner-A. A. Owens Dec. 21, 1992 DE Germany ........................ 4243 278.2 Attorney, Agent, or Firm-Sprung, Horn, Kramer & Jun. 28, 1993 DE Germany ........................ 43 21421.5 Woods 511 Int. Cl...................... A61K 31/44; C07D 213/64 57 ABSTRACT 52 U.S. C. ...................................... 514/345; 546/24; 546/290; 514/89 Substituted 4-phenyl-pyridones and 4-phenyl-2-alkox 58 Field of Search .................. 546/290, 24; 514/345, ypyridines are prepared by reducing corresponding 514/89 4-phenyl-pyridone and 4-phenyl-2-alkoxypyridine de rivatives. -

Chapter 13.Pptx
Chapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic O C C C N C N C X O nitro alkane X= F, Cl, Br, I amines Alkenes Alkyl Halide Chapter 23 OH C C H O C O C C O C C Alkynes phenol alcohols ethers epoxide acidic Chapter 14 H H H C S C C C C S S C C S C C H C C sulfides thiols disulfide H H (thioethers) Arenes 253 Nomenclature of alcohols 1. In general, alcohols are named in the same manner as alkanes; replace the -ane suffix for alkanes with an -ol for alcohols CH3CH2CH2CH3 CH3CH2CH2CH2OH OH butane 1-butanol 2-butanol butan-1-ol butan-2-ol 2. Number the carbon chain so that the hydroxyl group gets the lowest number 3. Number the substituents and write the name listing the substituents in alphabetical order. Many alcohols are named using non-systematic nomenclature H C OH 3 OH OH C OH OH HO OH H3C HO H3C benzyl alcohol allyl alcohol tert-butyl alcohol ethylene glycol glycerol (phenylmethanol) (2-propen-1-ol) (2-methyl-2-propanol) (1,2-ethanediol) (1,2,3-propanetriol) 254 127 Alcohols are classified according to the H R C OH C OH H H degree of substitution of the carbon bearing H H 1° carbon the -OH group methanol primary alcohol primary (1°) : one alkyl substituent R R C OH C OH R R secondary (2°) : two alkyl substituents H R 2° carbon 3° carbon tertiary (3°) : three alkyl substituents secondary alcohol tertiary alcohol Physical properties of alcohols – the C-OH bond of alcohols has a significant dipole moment. -

Radical Approaches to Alangium and Mitragyna Alkaloids
Radical Approaches to Alangium and Mitragyna Alkaloids A Thesis Submitted for a PhD University of York Department of Chemistry 2010 Matthew James Palframan Abstract The work presented in this thesis has focused on the development of novel and concise syntheses of Alangium and Mitragyna alkaloids, and especial approaches towards (±)-protoemetinol (a), which is a key precursor of a range of Alangium alkaloids such as psychotrine (b) and deoxytubulosine (c). The approaches include the use of a key radical cyclisation to form the tri-cyclic core. O O O N N N O O O H H H H H H O N NH N Protoemetinol OH HO a Psychotrine Deoxytubulosine b c Chapter 1 gives a general overview of radical chemistry and it focuses on the application of radical intermolecular and intramolecular reactions in synthesis. Consideration is given to the mediator of radical reactions from the classic organotin reagents, to more recently developed alternative hydrides. An overview of previous synthetic approaches to a range of Alangium and Mitragyna alkaloids is then explored. Chapter 2 follows on from previous work within our group, involving the use of phosphorus hydride radical addition reactions, to alkenes or dienes, followed by a subsequent Horner-Wadsworth-Emmons reaction. It was expected that the tri-cyclic core of the Alangium alkaloids could be prepared by cyclisation of a 1,7-diene, using a phosphorus hydride to afford the phosphonate or phosphonothioate, however this approach was unsuccessful and it highlighted some limitations of the methodology. Chapter 3 explores the radical and ionic chemistry of a range of silanes. -

Sodium Borohydride
Version 6.6 SAFETY DATA SHEET Revision Date 01/21/2021 Print Date 09/25/2021 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Sodium borohydride Product Number : 452882 Brand : Aldrich CAS-No. : 16940-66-2 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Synthesis of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) Chemicals which, in contact with water, emit flammable gases (Category 1), H260 Acute toxicity, Oral (Category 3), H301 Skin corrosion (Category 1B), H314 Serious eye damage (Category 1), H318 Reproductive toxicity (Category 1B), H360 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 GHS Label elements, including precautionary statements Pictogram Signal word Danger Aldrich - 452882 Page 1 of 9 The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the US and Canada Hazard statement(s) H260 In contact with water releases flammable gases which may ignite spontaneously. H301 Toxic if swallowed. H314 Causes severe skin burns and eye damage. H360 May damage fertility or the unborn child. -

United States Patent 0
1 3,667,923 United States Patent 0 ice Patented June 6, 1972 1 2 ‘I have discovered that anhydrous hydrogen cyanide $667,923 reacts readily with a quaternary ammonium borohydride PREPARATION OF LITHIUM, SODIUM AND and with the borohydrides of lithium and sodium at QUATERNARY AMMONIUM CYANOBORO atmospheric pressure in solvents for the borohydride, such HYDRIDES Robert C. Wade, Ipswich, Mass, assignor to Ventron as glyme, diglyme, triglyme, tetrahydrofuran and dimethyl Corporation, Beverly, Mass. formamide, or mixtures of these at a temperatures be No Drawing. Filed June 16, 1969, Ser. No. 833,722 tween 0° and the boiling point of the solvent to form Int. Cl. C01c 3/08; C01!) 35/00 the corresponding cyanoborohydride. The preferred sol US. Cl. 23-358 4 Claims vents are tetrahydrofuran and glyme because of their 10 convenient boiling points. Potassium borohydride does not react readily with ABSTRACT OF THE DISCLOSURE hydrogen cyanide in tetrahydrofuran or glyme presum The invention relates to the preparation of lithium, ably because of its lack of solubility in these solvents. sodium and quaternary ammonium cyanoborohydrides. However, it reacts with hydrogen cyanide in dimethyl These compounds are prepared by mixing substantially formamide similarly to the other borohydrides mentioned anhydrous hydrogen cyanide with a substantially an above. hydrous lithium or sodium or quaternary ammonium The reaction of the process of the invention appears to borohydride at a temperature between 0° C. and 100° C. take place in two stages. Thus, if the reaction mixture is in a substantially anhydrous solvent, such as tetrahydro initially maintained between about 10° and about 35° C. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

SAFETY DATA SHEET Revision Date 15.09.2021 According to Regulation (EC) No
Version 6.6 SAFETY DATA SHEET Revision Date 15.09.2021 according to Regulation (EC) No. 1907/2006 Print Date 24.09.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Lithium borohydride Product Number : 222356 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 16949-15-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Substances and mixtures which in contact with water emit flammable gases (Category 1), H260 Acute toxicity, Oral (Category 3), H301 Skin corrosion (Sub-category 1B), H314 For the full text of the H-Statements mentioned in this Section, see Section 16. Aldrich- 222356 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal word Danger Hazard statement(s) H260 In contact with water releases flammable gases which may ignite spontaneously. -

UNIT 6 ELEMENTS of GROUP 13 Structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction Uses I 6.3 General Characteristics
UNIT 6 ELEMENTS OF GROUP 13 structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction uses i 6.3 General Characteristics I - 6.5 Halides of Bpron anel Aluminium Halides of Boron Halides of Aluminium 6.6 Oxides of Boron and Aluminium I Boric Oxide Aluminium Oxide 6.7 Oxoacids of Boron and Borates 6.8 Borazine 6.9 Complexation Behaviour 6.10 Anomalous Behaviour of Boron 6.11 Summary 6.12 Terminal Questions 6.13- Answers ' -- 6.1 INTRODUCTION In the previous two units, you studied the main features of the chemistry of Group 1 and Group 2 elements, i.e. the alkali and the alkaline earth metals. In this unit you - will study the elements of Group 13, namely, boron, aluminium, gallium, indium and, thallium. While studying the alkali and alkaline earth metals, you have seen that all Zhe elements of these two groups are highly reactive metals and the first element of each group shows some differences from the rest. In Group 13, the differences between the first element and the remaining elements become so pronounced that the first member of the group, i.e. boron is a nonmetal wheieas the rest of the elements are distinctly metallic in nature. In a way, this is the first group of the periodic table in which you observe a marked change in the hature of the elements . down the group. describe the chemistry of hydrides, halides and oxides of boron and aluminium, elucidate the structures of hydrides of boron and aluminium, 6.2 OCCURRENC~,EXTRACTION AND USES Elements bf Group 13 are sufficiently reactive. -
![Reductive Amination with [11C]Formaldehyde: a Versatile Approach to Radiomethylation of Amines](https://docslib.b-cdn.net/cover/4140/reductive-amination-with-11c-formaldehyde-a-versatile-approach-to-radiomethylation-of-amines-684140.webp)
Reductive Amination with [11C]Formaldehyde: a Versatile Approach to Radiomethylation of Amines
International Journal of Organic Chemistry, 2012, 2, 202-223 http://dx.doi.org/10.4236/ijoc.2012.23030 Published Online September 2012 (http://www.SciRP.org/journal/ijoc) Reductive Amination with [11C]Formaldehyde: A Versatile Approach to Radiomethylation of Amines Chunying Wu1, Ruoshi Li1, Dorr Dearborn2, Yanming Wang1* 1Division of Radiopharmaceutical Science, Case Center for Imaging Research, Department of Radiology, Cleveland, USA 2Environmental Health, Case Western Reserve University, Cleveland, USA Email: *[email protected] Received February 16, 2012; revised March 24, 2012; accepted April 2, 2012 ABSTRACT Carbon-11 radiolabeled amines constitute a very important class of radioligands that are widely used for positron emis- 11 sion tomography (PET) imaging. Radiolabeling of amines is often achieved through radiomethylation using [ C]CH3I 11 or [ C]CH3OTf under basic conditions in a strictly anhydrous environment. Functional groups such as hydroxyl and carboxyl groups that are often present in the molecules are normally base sensitive and require protection and deprotec- tion, which substantially prolongs and complicates the radiolabeling process. Here we report a versatile approach to a series of C-11 radiolabeled amines prepared through reductive amination using [11C]formaldehyde. Using a variety of substrates bearing different functional groups, we demonstrate the general utility of this method. In contrast to conven- tional radiomethylation methods, the reductive amination using [11C]formaldehyde can be carried out in an aqueous environment relatively quickly without the need of protection of base-sensitive functional groups. Keywords: C-11 Formaldehyde; Radiomethylation; Reductive Amination; Positron Emission Tomography; Radiolabelling 1. Introduction probes must be labeled with positron-emitting radionu- clides such as carbon-11 or fluorine-18. -

Materials Chemistry Materials Chemistry
Materials Chemistry Materials Chemistry by Bradley D. Fahlman Central Michigan University, Mount Pleasant, MI, USA A C.I.P. Catalogue record for this book is available from the Library of Congress. ISBN 978-1-4020-6119-6 (HB) ISBN 978-1-4020-6120-2 (e-book) Published by Springer, P.O. Box 17, 3300 AA Dordrecht, The Netherlands. www.springer.com Printed on acid-free paper First published 2007 Reprinted 2008 All Rights Reserved © 2008 Springer Science + Business Media B.V. No part of this work may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording or otherwise, without written permission from the Publisher, with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. TABLE OF CONTENTS Preface ............................................................ ix Chapter 1. WHAT IS MATERIALS CHEMISTRY? .................... 1 1.1 HISTORICAL PERSPECTIVES . ............................ 2 1.2 CONSIDERATIONS IN THE DESIGN OF NEW MATERIALS . 5 1.3 DESIGN OF NEW MATERIALS THROUGH A “CRITICAL THINKING”APPROACH................................... 6 Chapter 2. SOLID-STATE CHEMISTRY ............................ 13 2.1 AMORPHOUS VS.CRYSTALLINESOLIDS.................. 13 2.2 TYPES OF BONDING IN SOLIDS . ..................... 14 2.2.1 IonicSolids......................................... 14 2.2.2 Metallic Solids . ................................ 16 2.2.3 Molecular Solids. ................................ 19 2.2.4 CovalentNetworkSolids.............................. 21 2.3 THECRYSTALLINESTATE................................ 21 2.3.1 Crystal Growth Techniques . ..................... 23 2.3.2 TheUnitCell ....................................... 25 2.3.3 CrystalLattices...................................... 28 2.3.4 CrystalImperfections................................. 41 2.3.5 Phase-Transformation Diagrams. ..................... 47 2.3.6 Crystal Symmetry and Space Groups . -

The Reaction of Sodium Borohydride
THE REACTION OF SODIUM BOROHYDRIDE WITH N-·ACYLANILINES By ROBERT FRA.."\\JK LINDEl"J.ANN Ir Bachelor of Arts Wittenberg College Springfield, Ohio 1952 Submitted to the Faculty of the Graduate School of the Oklahoma Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of MA.STER OF SCIENCE August, 19.54 i THE REACTION OF SODIUM BOROHYDRIDE WITH N-ACYLANILINES Thesis Approved: ~ - Dean of the Graduate School ii l!l Al C! 0~ 11J/~~. 2t"'I'Ii .ACKN01rJLEDGEMENT The author-wishes to express his gratitude to Dr. H. Po Johnston for the invaluable assistance and guidance given. This project was made possible by the Chemistry Department in the form of a Graduate Fellowship and by an unnamed sponsor through the Research Foundation. iii TABLE OF CONTENTS Page · I. .Introduction ...................................... ., o ••••••·· .... 1 II o Historical ... o o • .,, • (I .. ., •.••• G ••••.•••••••• .is •••••••• "' •. o o •••••• o '°. 2 Preparation of Sodium Borohydride ........................... 2 Physical Properties ofSodium Borohydrj.de ................... 3 Chemical Properties of · Sodium Borohydride .............. " •••• 3 III. Experimental. 0 ·• •• ·•. 0 •••• 0 ·• •••• 0. (I •••• 0 0 • 0 Q • .., ••• Q e. 0. 0. 0 D. 0 e 8 Purification of Sodium Borohydride ........................... 8 Reaction of Sodium Borohydride w:i. th Acetanilide ..........._ •• 9 Reaction of Sodium Borohydride w:i. th For.manilide oo •• oe.,. .... 27 Discussion ••• o o. o. o o o o. o ·O fl o • .e. o o ........ o. o. o o • ., o ~. o" o (Io o o o .29 IV. S11ITil11ary •• 0. Cl Ct. 0 0 0. 0. 0 -0 DO O O O C. c,.. 0. 0 4 11) 0 0 0 -0 0 0 0 -0 a O O .0 0 0 .0.