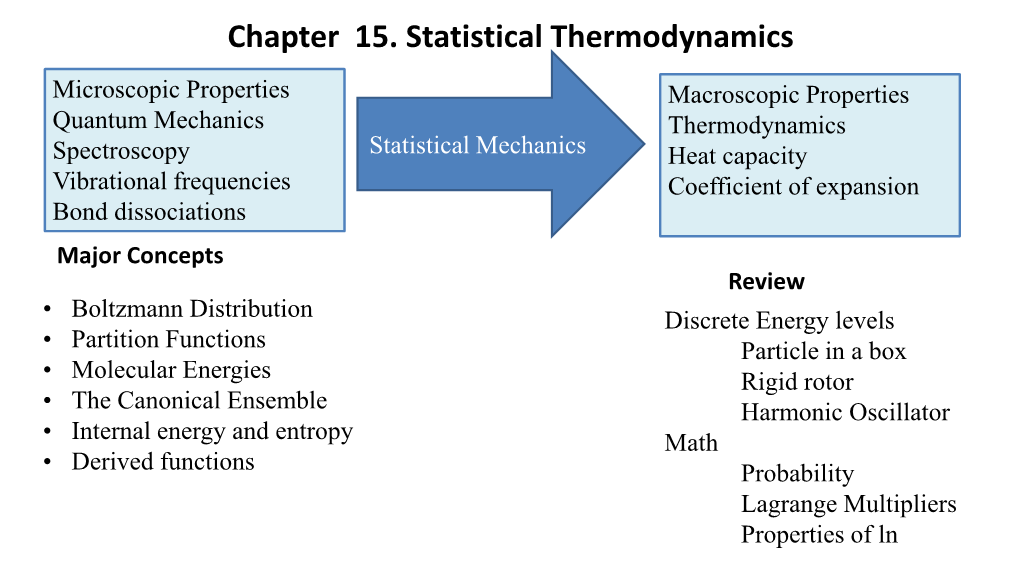
Chapter 15. Statistical Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -

Canonical Ensemble
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 8. Canonical Ensemble January 26, 2011 Contents Outline • In this chapter, we will establish the equilibrium statistical distribution for systems maintained at a constant temperature T , through thermal contact with a heat bath. • The resulting distribution is also called Boltzmann's distribution. • The canonical distribution also leads to definition of the partition function and an expression for Helmholtz free energy, analogous to Boltzmann's Entropy formula. • We will study energy fluctuation at constant temperature, and witness another fluctuation- dissipation theorem (FDT) and finally establish the equivalence of micro canonical ensemble and canonical ensemble in the thermodynamic limit. (We first met a mani- festation of FDT in diffusion as Einstein's relation.) Reading Assignment: Reif x6.1-6.7, x6.10 1 1 Temperature For an isolated system, with fixed N { number of particles, V { volume, E { total energy, it is most conveniently described by the microcanonical (NVE) ensemble, which is a uniform distribution between two constant energy surfaces. const E ≤ H(fq g; fp g) ≤ E + ∆E ρ (fq g; fp g) = i i (1) mc i i 0 otherwise Statistical mechanics also provides the expression for entropy S(N; V; E) = kB ln Ω. In thermodynamics, S(N; V; E) can be transformed to a more convenient form (by Legendre transform) of Helmholtz free energy A(N; V; T ), which correspond to a system with constant N; V and temperature T . Q: Does the transformation from N; V; E to N; V; T have a meaning in statistical mechanics? A: The ensemble of systems all at constant N; V; T is called the canonical NVT ensemble. -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action
Article A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action Ivan Kennedy 1,2,*, Harold Geering 2, Michael Rose 3 and Angus Crossan 2 1 Sydney Institute of Agriculture, University of Sydney, NSW 2006, Australia 2 QuickTest Technologies, PO Box 6285 North Ryde, NSW 2113, Australia; [email protected] (H.G.); [email protected] (A.C.) 3 NSW Department of Primary Industries, Wollongbar NSW 2447, Australia; [email protected] * Correspondence: [email protected]; Tel.: + 61-4-0794-9622 Received: 23 March 2019; Accepted: 26 April 2019; Published: 1 May 2019 Abstract: A convenient practical model for accurately estimating the total entropy (ΣSi) of atmospheric gases based on physical action is proposed. This realistic approach is fully consistent with statistical mechanics, but reinterprets its partition functions as measures of translational, rotational, and vibrational action or quantum states, to estimate the entropy. With all kinds of molecular action expressed as logarithmic functions, the total heat required for warming a chemical system from 0 K (ΣSiT) to a given temperature and pressure can be computed, yielding results identical with published experimental third law values of entropy. All thermodynamic properties of gases including entropy, enthalpy, Gibbs energy, and Helmholtz energy are directly estimated using simple algorithms based on simple molecular and physical properties, without resource to tables of standard values; both free energies are measures of quantum field states and of minimal statistical degeneracy, decreasing with temperature and declining density. We propose that this more realistic approach has heuristic value for thermodynamic computation of atmospheric profiles, based on steady state heat flows equilibrating with gravity. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

LECTURES on MATHEMATICAL STATISTICAL MECHANICS S. Adams
ISSN 0070-7414 Sgr´ıbhinn´ıInstiti´uid Ard-L´einnBhaile´ Atha´ Cliath Sraith. A. Uimh 30 Communications of the Dublin Institute for Advanced Studies Series A (Theoretical Physics), No. 30 LECTURES ON MATHEMATICAL STATISTICAL MECHANICS By S. Adams DUBLIN Institi´uid Ard-L´einnBhaile´ Atha´ Cliath Dublin Institute for Advanced Studies 2006 Contents 1 Introduction 1 2 Ergodic theory 2 2.1 Microscopic dynamics and time averages . .2 2.2 Boltzmann's heuristics and ergodic hypothesis . .8 2.3 Formal Response: Birkhoff and von Neumann ergodic theories9 2.4 Microcanonical measure . 13 3 Entropy 16 3.1 Probabilistic view on Boltzmann's entropy . 16 3.2 Shannon's entropy . 17 4 The Gibbs ensembles 20 4.1 The canonical Gibbs ensemble . 20 4.2 The Gibbs paradox . 26 4.3 The grandcanonical ensemble . 27 4.4 The "orthodicity problem" . 31 5 The Thermodynamic limit 33 5.1 Definition . 33 5.2 Thermodynamic function: Free energy . 37 5.3 Equivalence of ensembles . 42 6 Gibbs measures 44 6.1 Definition . 44 6.2 The one-dimensional Ising model . 47 6.3 Symmetry and symmetry breaking . 51 6.4 The Ising ferromagnet in two dimensions . 52 6.5 Extreme Gibbs measures . 57 6.6 Uniqueness . 58 6.7 Ergodicity . 60 7 A variational characterisation of Gibbs measures 62 8 Large deviations theory 68 8.1 Motivation . 68 8.2 Definition . 70 8.3 Some results for Gibbs measures . 72 i 9 Models 73 9.1 Lattice Gases . 74 9.2 Magnetic Models . 75 9.3 Curie-Weiss model . 77 9.4 Continuous Ising model . -

Entropy and H Theorem the Mathematical Legacy of Ludwig Boltzmann
ENTROPY AND H THEOREM THE MATHEMATICAL LEGACY OF LUDWIG BOLTZMANN Cambridge/Newton Institute, 15 November 2010 C´edric Villani University of Lyon & Institut Henri Poincar´e(Paris) Cutting-edge physics at the end of nineteenth century Long-time behavior of a (dilute) classical gas Take many (say 1020) small hard balls, bouncing against each other, in a box Let the gas evolve according to Newton’s equations Prediction by Maxwell and Boltzmann The distribution function is asymptotically Gaussian v 2 f(t, x, v) a exp | | as t ≃ − 2T → ∞ Based on four major conceptual advances 1865-1875 Major modelling advance: Boltzmann equation • Major mathematical advance: the statistical entropy • Major physical advance: macroscopic irreversibility • Major PDE advance: qualitative functional study • Let us review these advances = journey around centennial scientific problems ⇒ The Boltzmann equation Models rarefied gases (Maxwell 1865, Boltzmann 1872) f(t, x, v) : density of particles in (x, v) space at time t f(t, x, v) dxdv = fraction of mass in dxdv The Boltzmann equation (without boundaries) Unknown = time-dependent distribution f(t, x, v): ∂f 3 ∂f + v = Q(f,f) = ∂t i ∂x Xi=1 i ′ ′ B(v v∗,σ) f(t, x, v )f(t, x, v∗) f(t, x, v)f(t, x, v∗) dv∗ dσ R3 2 − − Z v∗ ZS h i The Boltzmann equation (without boundaries) Unknown = time-dependent distribution f(t, x, v): ∂f 3 ∂f + v = Q(f,f) = ∂t i ∂x Xi=1 i ′ ′ B(v v∗,σ) f(t, x, v )f(t, x, v∗) f(t, x, v)f(t, x, v∗) dv∗ dσ R3 2 − − Z v∗ ZS h i The Boltzmann equation (without boundaries) Unknown = time-dependent distribution -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

Spin Statistics, Partition Functions and Network Entropy
This is a repository copy of Spin Statistics, Partition Functions and Network Entropy. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/118694/ Version: Accepted Version Article: Wang, Jianjia, Wilson, Richard Charles orcid.org/0000-0001-7265-3033 and Hancock, Edwin R orcid.org/0000-0003-4496-2028 (2017) Spin Statistics, Partition Functions and Network Entropy. Journal of Complex Networks. pp. 1-25. ISSN 2051-1329 https://doi.org/10.1093/comnet/cnx017 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ IMA Journal of Complex Networks (2017)Page 1 of 25 doi:10.1093/comnet/xxx000 Spin Statistics, Partition Functions and Network Entropy JIANJIA WANG∗ Department of Computer Science,University of York, York, YO10 5DD, UK ∗[email protected] RICHARD C. WILSON Department of Computer Science,University of York, York, YO10 5DD, UK AND EDWIN R. HANCOCK Department of Computer Science,University of York, York, YO10 5DD, UK [Received on 2 May 2017] This paper explores the thermodynamic characterization of networks using the heat bath analogy when the energy states are occupied under different spin statistics, specified by a partition function. -

A Molecular Modeler's Guide to Statistical Mechanics
A Molecular Modeler’s Guide to Statistical Mechanics Course notes for BIOE575 Daniel A. Beard Department of Bioengineering University of Washington Box 3552255 [email protected] (206) 685 9891 April 11, 2001 Contents 1 Basic Principles and the Microcanonical Ensemble 2 1.1 Classical Laws of Motion . 2 1.2 Ensembles and Thermodynamics . 3 1.2.1 An Ensembles of Particles . 3 1.2.2 Microscopic Thermodynamics . 4 1.2.3 Formalism for Classical Systems . 7 1.3 Example Problem: Classical Ideal Gas . 8 1.4 Example Problem: Quantum Ideal Gas . 10 2 Canonical Ensemble and Equipartition 15 2.1 The Canonical Distribution . 15 2.1.1 A Derivation . 15 2.1.2 Another Derivation . 16 2.1.3 One More Derivation . 17 2.2 More Thermodynamics . 19 2.3 Formalism for Classical Systems . 20 2.4 Equipartition . 20 2.5 Example Problem: Harmonic Oscillators and Blackbody Radiation . 21 2.5.1 Classical Oscillator . 22 2.5.2 Quantum Oscillator . 22 2.5.3 Blackbody Radiation . 23 2.6 Example Application: Poisson-Boltzmann Theory . 24 2.7 Brief Introduction to the Grand Canonical Ensemble . 25 3 Brownian Motion, Fokker-Planck Equations, and the Fluctuation-Dissipation Theo- rem 27 3.1 One-Dimensional Langevin Equation and Fluctuation- Dissipation Theorem . 27 3.2 Fokker-Planck Equation . 29 3.3 Brownian Motion of Several Particles . 30 3.4 Fluctuation-Dissipation and Brownian Dynamics . 32 1 Chapter 1 Basic Principles and the Microcanonical Ensemble The first part of this course will consist of an introduction to the basic principles of statistical mechanics (or statistical physics) which is the set of theoretical techniques used to understand microscopic systems and how microscopic behavior is reflected on the macroscopic scale.