Study on Yearly Variation of Physico-Chemical Parameters Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Montecarlo E-Magazine Dec20 Proof
Issue – 9: October – December 2020 Welcome Happy New Year 2021 “ Learn from yesterday, Live for today, Hope for tomorrow.” 2. Message from JMD’s Desk 3. Message from Editor’s Desk 4. Felicitation of Long Services of Our Employees 5. Festival Celebration at HO Ahmedabad 6. Festival Celebration at Sites 7. Project Milestone 9. QUIZ 10. Know Your Senior Leader 12. Journey of Human Resource Excellence 13. Mobile Applications & IT Tools 16. PPE Awareness Campaign at Sites 17. Salient Features of the Group Term Assurance Plan 18. Covid – 19 Warriors Experiences 20. Employee Corner 22. Words of Appreciation MONTECARLO MESSAGE FROM JMD’S DESK Dear Montecarlo Family Members, New Year Greetings! It is my pleasure to connect with you all through this platform. I hope that you and your loved ones are safe and healthy. We all agree that this pandemic has drastically impacted our way of living and work. I am proud and inspired by the way our company has risen to this challenge with flexibility, cour- age, and a caring heart. Thank you for your enormous contributions. Let’s work together across all parts of the business to keep going. To capitalize on today's greatest changes and to identify emerging trends, innovation is more essential than ever. Improving operations and technology integration are important. To offset the fierce competition in the market, all of us are required to be more cost-conscious and optimize the usage of all our resources ensuring the quality and safety of our operations. For the Financial Year ended on 31st March 2020, we have recorded a turnover of Rs. -

Final English Version of E-Bulletin (6
Embassy of India 6 - 12 Dec 2020 Jakarta INDIA'S LATEST NEWS NEWS MISSION'S ACTIVITIES HIGHLIGHT Agra, City of Taj Mahal Metro project inaugurated; Nitin Gadkari Inaugurates Three- Lane Koilwar Bridge on Sone River Worth Rs 266 Crore; Bhagalpur address for winged guests; #India@75 Efficient new method of harvesting water from air developed by IIT Guwahati; Lakshadweep declared first UT to become 100% organic. : indianembassyjakarta.gov.in : IndianEmbJkt : IndiaInIndonesia : Embassy of India Jakarta 1 Agra, City of Taj Mahal Metro project inaugurated Prime Minister Narendra Modi inaugurated the construction work of Agra Metro project in Agra, Uttar Pradesh through video conference. The metro project comprised of two corridors with a total length of 29.4 km and is estimated to cost Rs 8,3796.2 Million. The project will cater to more than 6 million tourists and 2.6 million residents of Agra every year. It will also provide an environment-friendly mass rapid transit system to Agra. The project will connect major tourist attractions like Taj Mahal, Agra Fort, Sikandra with railway stations and bus stands and it will be completed in five years. The metro in Agra will run at a speed of 80 Kmph and the cost of one metro rail of three coaches is pegged at Rs 80 Million. Source: Times Now Nitin Gadkari Inaugurates Three-Lane Koilwar Bridge on Sone River Worth Rs 266 Crore Union Minister for Road Transport and Highways, Nitin Gadkari, inaugurated the three-lane Koilwar bridge on the Sone river in Bihar through video conference. The bridge costing Rs 266 crore would make commuting easier in South Bihar. -

Dear Sir, with Vast Network of Deep & Wide Perennial Rivers, Cities Situated
Dear Sir, With vast network of deep & wide perennial rivers, cities situated on river banks & densely populated masses; construction of bridge infrastructure is the need of the hour. But on the front of design & durability, it can be gauged by the fact that bridges in India are designed to withstand 50 floods whereas it is 150 in the developed countries. In India, the aging period of bridges is 30 to 40 years. But there are prominent bridges more than 100 years old and had been constructed during the British era. As the bridges and the expressways they span keep rising, construction has become an end unto itself. Fueled by government-backed loans and urged on by the big construction companies and officials who profit from them, many of the projects are piling up debt and breeding corruption while producing questionable transportation benefits. As a developing nation, rapid development of transport infrastructure is happening in India like some other developing countries. The eye-popping structures have slashed travel times in some areas, made business easier and generated a sizable slice of the country’s economy, laying a foundation, in theory at least, for decades of future growth. But as an Engineer my concern is about development/ construction of robust & durable transport infrastructures .The shortsightedness of development policies are gnawing at the foundations of bridges in the country. Selection of construction agencies is more of the client’s requirement but where its implementation is concerned, where supervision & quality assurance is concerned, our role is important and crucial one. There are innumerable shortcomings on supervision & quality assurance’s fronts but one that relates to the selections & policy frameworks & which from my core of my heart I want that Authorities should act on it as follows: One of the factors for poor inspection standards is the age of Supervision Engineer. -

December 2020
Visit: www.civilstaphimachal.com For Any Query Mail us: [email protected] or call us at - (+91)-7814622609 1 Contents National News ............................................................................................................................................................ 16 1.1 ‘Ramayan Cruise Service’ to be launched in Ayodhya, Uttar Pradesh ....................................................... 16 1.2 Shri Dharmendra Pradhan launches world-class premium grade Petrol- XP100 ....................................... 16 1.3 Shri Dharmendra Pradhan inaugurates MNGL’s 100th CNG station .......................................................... 17 1.4 IIT-Ropar & IIT Alumni Council partners to set up Global Centre of Excellence ......................................... 17 1.5 Nagaland celebrates Hornbill Festival 2020 ............................................................................................... 17 1.6 Oman issues directive to establish Oman-India Friendship Association .................................................... 17 1.7 Shri Dharmendra Pradhan launches India’s first mobile CNG dispensing unit .......................................... 17 1.8 Bengaluru to host India’s first AI-enabled conversational digital persona ................................................ 17 1.9 RIL tops Fortune 500 list of Indian companies ........................................................................................... 17 1.10 India signs MoU with USA on Intellectual Property cooperation .............................................................. -
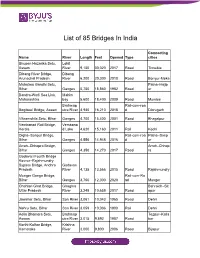
List of 85 Bridges in India
List of 85 Bridges In India Connecting Name River Length Feet Opened Type cities Bhupen Hazarika Setu, Lohit Assam River 9,150 30,020 2017 Road Tinsukia Dibang River Bridge, Dibang Arunachal Pradesh River 6,200 20,300 2018 Road Bomjur-Meka Mahatma Gandhi Setu, Patna–Hajip Bihar Ganges 5,750 18,860 1982 Road ur Bandra-Worli Sea Link, Mahim Maharashtra bay 5,600 18,400 2009 Road Mumbai Brahmap Rail-cum-roa Bogibeel Bridge, Assam utra River 4,940 16,210 2018 d Dibrugarh Vikramshila Setu, Bihar Ganges 4,700 15,400 2001 Road Bhagalpur Vembanad Rail Bridge, Vembana Kerala d Lake 4,620 15,160 2011 Rail Kochi Digha–Sonpur Bridge, Rail-cum-roa Patna–Sonp Bihar Ganges 4,556 14,948 2016 d ur Arrah–Chhapra Bridge, Arrah–Chhap Bihar Ganges 4,350 14,270 2017 Road ra Godavari Fourth Bridge Kovvur–Rajahmundry Bypass Bridge, Andhra Godavari Pradesh River 4,135 13,566 2015 Road Rajahmundry Munger Ganga Bridge, Rail-cum-Ro Bihar Ganges 3,750 12,300 2020 ad Munger Chahlari Ghat Bridge, Ghaghra Bahraich–Sit Uttar Pradesh River 3,249 10,659 2017 Road apur Jawahar Setu, Bihar Son River 3,061 10,043 1965 Road Dehri Nehru Setu, Bihar Son River 3,059 10,036 1900 Rail Dehri Kolia Bhomora Setu, Brahmap Tezpur–Kalia Assam utra River 3,015 9,892 1987 Road bor Korthi-Kolhar Bridge, Krishna Karnataka River 3,000 9,800 2006 Road Bijapur Netaji Subhas Chandra Kathajodi Bose Setu, Odisha River 2,880 9,450 2017 Road Cuttack Godavari Bridge, Andhra Godavari Rail-cum-roa Pradesh River 2,790 1974 d Rajahmundry Old Godavari Bridge Now decommissioned, Godavari Andhra Pradesh -

BIHAR SPECIAL Current Affairs (December 2020)
www.gradeup.co BIHAR SPECIAL Current Affairs (December 2020) Bollywood actor Sanjay Mishra becomes brand ambassador of Patna Municipal Corporation • The people of Bihar to make the state capital clean and beautiful, Bollywood’s famous comedy actor Sanjay Mishra has been declared the brand ambassador of Patna Municipal Corporation. Actor Sanjay Mishra who is going to convey the message, “Beauty of Patna is the Duty of Patna” among the people the state capital. Kabartal Wetland of Begusarai included in the Ramsar list • Ramsar Convention declared Kabartal Wetland also known as Kanwar Jheel in Begusarai, Bihar as a site of international importance. It covers 2,620 hectares of the Indo-Gangetic plains in the northern Bihar State. • Kabartal has become the first in Bihar and the 39th Ramsar site in India. This lake was given the status of bird sanctuary by the Bihar government in 1987. EC Appoints First Transgender Woman as Presiding Officer for Bihar Polls • Monika Das, is the first-ever transgender person to be appointed as a presiding poll officer in independent India’s history. Her selection is a big victory for the transgender community, which can often be underrepresented in public life. • For the past five years, she has been working at a bank, currently employed with Canara Bank. Prior to this, Monica has also become the country's first transgender banker. Fisheries and animal husbandry schemes inaugurated in Bihar • Prime Minister Shri Narendra Modi launched PM Matsya Sampada Yojana, e- Gopala App & several initiatives linked to studies and research in fisheries production, dairy, animal husbandry and agriculture in Bihar through video conferencing. -

Final Feasibility Report on Detailed Hydrographic Survey Sone River
STRABAG India Pvt Ltd, New Delhi FINAL FEASIBILITY REPORT ON DETAILED HYDROGRAPHIC SURVEY SONE RIVER FROM GANGA CONFLUENCE AT TODARPUR (CH 0 KM), TO INDRAPURI BARRAGE NEAR DEHRI (CH 141.0 KM) NATIONAL WATERWAY NO- 94 VOLUME-I Submitted To INLAND WATERWAYS AUTHORITY OF INDIA A-13, Sector-1,NOIDA DIST-Gautam Buddha Nagar UTTAR PRADESH PIN- 201 301(UP) Email: [email protected] Web: www.iwai.nic.in Submitted By STRABAG INDIA PVT LTD. Southern Park, Unit No.6, 4th Floor D-2, DDA District Centre, Saket, New Delhi 110017 India Tel : 91-11 492 01 492 , 91-11 492 01 454 Fax : 91-11 -492 01 493 Email: [email protected] Web:www.strabagindia.com Submitted Date / 09 /2018 Date of Submission: /01 /2018 IWAI - NW-94, Sone River (Todarpur to Indrapuri barrage near Dehri) P a g e | 1 STRABAG India Pvt Ltd, New Delhi ACKNOWLEDGEMENT STRABAG India Pvt. Ltd. (SIPL) express their gratitude to Shri S. K. Gangwar, Member (Technical) and Ms. Nutan Guha Biswas, IAS, Ex-Chairperson, IWAI for providing us the opportunity for "Detailed Hydrographic and Topography Survey of Sone River in Region VII of Proposed new waterways, a dream and very important part of Nation Development”. SIPL would also like to thank Shri Pravir Pandey, IA & AS, Chairman, Shri Alok Ranjan, ICAS, Member (Finance) and Shri Shashi Bhushan Shukla, Member (Traffic). SIPL wishes to express their gratitude to Capt. Ashish Arya, Hydrographic Chief and Cdr. P.K. Srivastava, Ex Hydrographic Chief, IWAI for his guidance and technical inputs in this project. SIPL would also like to thank Sh. -

Annual Progress Report (2013- 2014)
ANNUAL PROGRESS REPORT (2013- 2014) PRAGATI GRAMIN VIKAS SAMITI PRAGATI GRAMIN VIKAS SAMITI PRAGATI BHAWAN, RAMJAIPAL NAGAR, WEST BAILEY ROAD, PATNA-801503, BIHAR, INDIA Tel-0612-6590425, 9431017138, 8544060901 [email protected] Foreword Dear well-wishers and friends, This gives me great pleasure to present the Annual Report2013-14. As many of you know, Pragati Gramin Vikas Samiti is a community development organization, dedicated to help the poor, needy, deprived, under-privileged and vulnerable section of the society. At present we are working on issues like Water & Sanitation, Education, women empowerment, Land rights and Livelihood. The essence of our programme is that people themselves must participate, with increasing awareness and sense of responsibility, in the planning and implementation of project activities to their own well-being. PGVS believes that development is a process of socio-economic progress, which is best achieved through active participation of the people. PGVS emphasizes building on the resources of the people by increasing their knowledge, skills and economic strength thereby enabling them to manage the issues affecting their lives. The efforts are towards conscientious development process. Women’s issues and problems remain in the core of our concerns and their empowerment is integrated in all our programmes. We invite you to remain in touch and help us in our endeavors. With best wishes, Pradeep Priyadarshi Secretary PRAGATI GRAMIN VIKAS SAMITI Pragati Gramin Vikas Samiti: An Introduction Pragati Gramin Vikas Samiti is a non- profit, humanitarian voluntry organization dedicated to help the poor, needy,deprived, under- privileged and vulnerable section of the society. PGVS believes that development is a process of socio-economic progress, which is best achieved through active participation of the community people. -

Legal Fortnight December Edition Volume
1 FOREWORD More has been said about the writing of lawyers and judges than of any other group, except, of course, poets and novelists. The difference is that while the latter has usually been admired for their writing, the public has almost always damned lawyers and judges for theirs. If this state of affairs has changed in recent times, it is only in that many lawyers and judges have now joined the rest of the world is complaining about the quality of legal prose. My best wishes to all these student contributors, for their future endeavors. My best wishes and assurance to the readers that this will add a lot to the knowledge after reading this perfect case compilation. It’s not just for the legal fraternity but for anyone who has an interest in the field of law. By Vrinda Khanna & Nandini Mangla 2 PREFACE All India Legal Forum is replenished with information to give students a ready reference to the various areas of legal issues and news. All India Legal Forum is a team of more than 400 law students across the country to tackle basic problems which a legal researcher faces in day to day life, putting forward the basic things needed for researching and drafting. The All India Legal forum strives at providing a valuable contribution to contemporary legal issues and development. The organization seeks to bring out a platform to provide resourceful insights on law-related topics for the ever-growing legal fraternity. All India Legal Forum doesn't just publish blogs but also guides the authors. -

Bihar in Figures
BIHAR December 2020 For updated information, please visit www.ibef.org Table of Contents Executive Summary 3 Introduction 4 Economic Snapshot 9 Physical Infrastructure 15 Social Infrastructure 23 Industrial Infrastructure 26 Key Sectors 28 Key Procedures & Policies 35 Appendix 43 2 Executive summary 1 Fastest growing state 5 Third largest mobile in India subscriber base . With 84.22 million mobile subscribers in • The Gross State Domestic Product (GSDP) of Bihar increased at a CAGR January 2020, Bihar* accounted for the of 13.27% from 2015 to 2020 and is 5 third largest mobile subscriber base among projected to register a CAGR of 13.17% all the Indian states. between 2015 and 2021. 1 2 Fastest per capita 4 Strong agriculture income growth 2 4 sector . Bihar has witnessed strong growth in • Bihar has high agricultural production per capita net state domestic product. making it one of the strongest sectors At current prices, per capita NSDP of of the state. About 80% of the state’s the state grew at a CAGR of 13.41% (in population is employed in agriculture, which is much higher as compared to Rs.) between 2015 and 2021. 3 India’s average. The state has . developed as the number one state in the eastern region in terms of 3 Leader in the production of agricultural production. fruits and vegetables • Bihar is the fourth largest producer of vegetables and the eighth largest producer of fruits in India. Note: * - includes Jharkhand, Source: Central Statistics Office, Ministry of Statistics and Programme Implementation, Ministry for Integrated Development of Horticulture, NSDP- Net State Domestic Product 3 INTRODUCTION 4 Bihar fact file Patna Capital 1,106 persons per sq km 120,590,000 Population density total population 94,163 sq.km. -

National News
OAS PRELIMS 2020 VST 1 WWW.OBJECTIVEIAS.IN NATIONAL NEWS OAS PRELIMS 2020 CURRENT AFFAIRS January 2020 • National Stock Exchange (NSE) Knowledge Hub inaugurated in New Delhi. The Hub will assist the banking, financial services and insurance (BFSI) sector. • The Cabinet Committee on Economic Affairs (CCEA) has given its ‘in principle’ approval for strategic disinvestment of 4 Central Public Sector Enterprises (CPSEs) namely Minerals & Metals Trading Corporation (MMTC), MECON, Bharat Heavy Electricals (BHEL) and National Mineral Development Corporation (NMDC) & 2 Odisha State PSUs namely Industrial Promotion and Investment Corporation of Odisha (IPICOL) and Odisha Mining Corporation (OMC) in Neelachal Ispat Nigam Limited (NINL). • The union cabinet has granted the approval of giving the status of Institution of National Importance to a cluster of Ayurvedic institutions at Gujarat Ayurved University in Jamnagar. Jamnagar Ayurveda University will become the 1st Ayurveda institute to join the ranks of the IIT’s and AIIMS. • The Union Government has authorized the amount of Rs 5559 crore as Viability Gap Funding (VGF) or 1656 Km long Indra-dhanush Natural Gas Pipeline Grid project. The project will connect 8 North Eastern states: Assam, Arunachal Pradesh, Meghalaya, Manipur, Mizoram, Nagaland, Tripura and Sikkim. • Indian Railways is in the process of installing Internet Protocol (IP) based Video Surveillance System (VSS) at stations to enhance security. • Census exercise India-2021 to begin on 1st April 2020 and will end on 30th of September 2020. It will be conducted through a mobile phone application. • The “Paperless Licensing Process” has been launched by the Department for Promotion of Industry and Internal Trade (DPIIT), for Petroleum Road Tankers. -

PRE-Mix December 2020
PRE-Mix (Compilations of the Multiple Choice Questions) For the Month Of December 2020 Visit our website www.sleepyclasses.com or our YouTube channel for entire GS Course FREE of cost Also Available: Prelims Crash Course || Prelims Test Series T.me/SleepyClasses Table of Contents 1. Geography ...........................................................................................................1 2. History & Culture .............................................................................................19 3. Polity & Governance .......................................................................................37 4. Economy ..............................................................................................................56 5. Environment & Ecology .................................................................................75 6. Science & Technology .....................................................................................93 www.sleepyclasses.com Call 6280133177 T.me/SleepyClasses 1. Geography Click on the links given below to watch the following questions on YouTube • Video 1 • Video 2 • Video 3 • Video 4 1. Shahtoot dam, recently heard in news, is in A. Pakistan B. Afghanistan C. India D. Bhutan Answer: B Explanation • India will be constructing the Shahtoot Dam on Kabul River in Afghanistan and that the Governments of two nations have recently concluded an agreement for the same. • The dam's construction would provide safe drinking water to two million residents of Kabul city which is the Afghan