ZHONG-THESIS-2016.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pindolol of the Activation of Postsynaptic 5-HT1A Receptors
Potentiation by (-)Pindolol of the Activation of Postsynaptic 5-HT1A Receptors Induced by Venlafaxine Jean-Claude Béïque, Ph.D., Pierre Blier, M.D., Ph.D., Claude de Montigny, M.D., Ph.D., and Guy Debonnel, M.D. The increase of extracellular 5-HT in brain terminal regions antagonist WAY 100635 (100 g/kg, i.v.). A short-term produced by the acute administration of 5-HT reuptake treatment with VLX (20 mg/kg/day ϫ 2 days) resulted in a inhibitors (SSRI’s) is hampered by the activation of ca. 90% suppression of the firing activity of 5-HT neurons somatodendritic 5-HT1A autoreceptors in the raphe nuclei. in the dorsal raphe nucleus. This was prevented by the The present in vivo electrophysiological studies were coadministration of (-)pindolol (15 mg/kg/day ϫ 2 days). undertaken, in the rat, to assess the effects of the Taken together, these results indicate that (-)pindolol coadministration of venlafaxine, a dual 5-HT/NE reuptake potentiated the activation of postsynaptic 5-HT1A receptors inhibitor, and (-)pindolol on pre- and postsynaptic 5-HT1A resulting from 5-HT reuptake inhibition probably by receptor function. The acute administration of venlafaxine blocking the somatodendritic 5-HT1A autoreceptor, but not and of the SSRI paroxetine (5 mg/kg, i.v.) induced a its postsynaptic congener. These results support and extend suppression of the firing activity of dorsal hippocampus CA3 previous findings providing a biological substratum for the pyramidal neurons. This effect of venlafaxine was markedly efficacy of pindolol as an accelerating strategy in major potentiated by a pretreatment with (-)pindolol (15 mg/kg, depression. -

Strategies for Managing Sexual Dysfunction Induced by Antidepressant Medication
King’s Research Portal DOI: 10.1002/14651858.CD003382.pub3 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Taylor, M. J., Rudkin, L., Bullemor-Day, P., Lubin, J., Chukwujekwu, C., & Hawton, K. (2013). Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database of Systematic Reviews, (5). https://doi.org/10.1002/14651858.CD003382.pub3 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

Flibanserin (Addyi)
STEPS New Drug Reviews Flibanserin (Addyi) for Hypoactive Sexual Desire Disorder in Premenopausal Women HARRY HOLT, MD, and JEFFREY TINGEN, PharmD, MBA, University of Virginia Health System, Charlottesville, Virginia STEPS new drug reviews Flibanserin (Addyi) is labeled for the treatment of acquired, generalized hypoactive sexual cover Safety, Tolerability, desire disorder (HSDD) in premenopausal women. It is a nonhormonal medication that Effectiveness, Price, and 1 Simplicity. Each indepen- affects serotonin receptors to increase libido. dent review is provided by authors who have no financial association with Drug Dosage Dose form Cost* the drug manufacturer. Flibanserin (Addyi) 100 mg per day at bedtime 100-mg tablet $830 This series is coordinated by Allen F. Shaughnessy, *—Estimated retail price of one month’s treatment based on information obtained at http://www.goodrx.com PharmD, MMedEd, (accessed April 1, 2016). Contributing Editor. A collection of STEPS pub- lished in AFP is available at http://www.aafp.org/ SAFETY EFFECTIVENESS afp/steps. The main risks of flibanserin are hypoten- Flibanserin has been evaluated in three ran- sion (2%) and syncope (0.4%), which are domized, double-blind, placebo-controlled more likely to occur in patients who have studies of 2,375 premenopausal women also ingested alcohol. For this reason, women with acquired, generalized HSDD, defined should not drink alcohol when taking fliban- as low sexual desire causing marked dis- serin; about one in six persons taking this tress or interpersonal difficulties.2 Women combination will experience clinically signif- in monogamous, heterosexual relationships icant hypotension and syncope.1 Flibanserin with no known cause of HSDD reported should not be taken by women with hepatic an average increase of 1.6 to 2.5 additional impairment or women who are also taking satisfying sexual events per month with treat- moderate or strong cytochrome P450 3A4 ment, from a baseline of 2.5 to 3.0 per month. -

Dosing and Monitoring: Children and Adolescents
Dosing and Monitoring: Children and Adolescents Glenn S. Hirsch, MD Keith S. Ditkowski, MD Child & Adolescent Dosing.indd 1 08-01-2018 14:56:52 Adapted from Child & Adolescent Dosing.indd 76 08-01-2018 14:56:50 DISCLAIMER This pocket reference is provided as a service to medicine by the publisher, Medworks Media Inc. This review does not imply the publisher’s agreement with the views expressed herein. Although every effort has been made to ensure that drug doses and other information are presented accurately in this publication, the ultimate responsibility rests with the prescribing physician. Neither the publisher, nor the authors can be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound currently under clinical investigation. In an effort to allow for the widest distribution of these guidelines, the authors have modified the originally printed material to more closely conform to the limitations of product labeling. For many of the drugs discussed herein, initiation at lower doses may increase tolerability and efficacy. Copyright ©2018, MedWorks Media Inc., 2205 Rockefeller Lane, Redondo Beach, CA 90278. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form. Child & Adolescent Dosing.indd 2 08-01-2018 14:56:52 Dosing and Monitoring: Children and Adolescents Glenn S. Hirsch, MD Keith S. Ditkowski, MD Dr. Hirsch is the deputy director of the New York University Child Study Center, the medical director in the Division of Child and Adolescent Psychiatry, and the assistant professor of psychiatry at the New York University School of Medicine. -

(Flibanserin) (Flibanserin) Tablets
™ MEDICATION GUIDE addyi ADDYI™ (add-ee) (flibanserin) (flibanserin) Tablets Read this Medication Guide before you start taking ADDYI™ and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor. What is the most important information I should know about ADDYI? Your risk of severe low blood pressure and fainting (loss of consciousness) is increased if you take ADDYI and: • drink alcohol. Do not drink alcohol if you take ADDYI. • take certain prescription medicines, over-the-counter medicines, or herbal supplements. Do not take or start taking any prescription medicines, over-the-counter medicines, or herbal supplements while taking ADDYI until you have talked with your doctor. Your doctor will tell you if it is safe to take other medicines or herbal supplements while you are taking ADDYI. • have liver problems. Do not take ADDYI if you have liver problems. If you take ADDYI and you feel lightheaded or dizzy, lie down right away. Get emergency medical help or ask someone to get emergency medical help for you if the symptoms do not go away or if you faint (lose consciousness). If you faint (lose consciousness), tell your doctor as soon as you can. ADDYI is only available through the ADDYI Risk Evaluation and Mitigation Strategy (REMS) Program because of the increased risk of severe low blood pressure and fainting (loss of consciousness) with alcohol use. You can only get ADDYI from pharmacies that are enrolled in the ADDYI REMS Program. For more information about the Program and a list of pharmacies that are enrolled in the ADDYI REMS Program, go to www.AddyiREMS.com or call 1-844-PINK-PILL (1-844- 746-5745). -

(12) United States Patent (10) Patent No.: US 7,776,345 B2 Dudhara Et Al
USOO7776345B2 (12) United States Patent (10) Patent No.: US 7,776,345 B2 Dudhara et al. (45) Date of Patent: Aug. 17, 2010 (54) GASTRIC RETENTION CONTROLLED DRUG 4,996,058 A 2f1991 Sinnreich DELIVERY SYSTEM 5,091, 184 A 2, 1992 Khanna ...................... 424/435 5,096,714 A 3, 1992 Kuhrts (75) Inventors: Kamlesh Mohanlal Dudhara, Baroda 5,651,985 A 7/1997 Penners et al. 5,654,005 A * 8/1997 Chen et al. .................. 424/480 (IN); Nitin Bhalachandra 6,261,601 B1* 7/2001 Talwar et al. ... ... 424/469 Dharmadhikari, Mumbai (IN); Vaishali 6,340.475 B2 * 1/2002 Shell et al. .................. 424/469 Vijay Dhavse, Mumbai (IN) 6,960,356 B1 1 1/2005 Talwar et al. 7,109,239 B2 * 9/2006 Gallop et al. ............... 514,533 (73) Assignee: Sun Pharma Advanced Research 2007/0265343 A1 11/2007 Dharmadhikari et al. Company Ltd, Andheri (East) (IN) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this EP 1095650 5, 2001 patent is extended or adjusted under 35 JP 630.14715 1, 1988 U.S.C. 154(b) by 1759 days. WO WO 98.51408 11, 1998 WO 99.47 128 A1 9, 1999 (21) Appl. No.: 10/482,770 WO OO15198 A1 3, 2000 WO WOOOf 15198 3, 2000 (22) PCT Filed: Jul. 4, 2002 WO WOOO, 23045 4/2000 WO WOOO,386.50 * 7/2OOO (86). PCT No.: PCT/NO2AOO144 WO 01/08670 A2 2, 2001 WO WO 01/10417 A1 2, 2001 S371 (c)(1), WO WO 01/10419 A1 2, 2001 (2), (4) Date: Dec. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Interactions with HBV Treatment
www.hep-druginteractions.org Interactions with HBV Treatment Charts revised September 2021. Full information available at www.hep-druginteractions.org Page 1 of 6 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. ADV, Adefovir; ETV, Entecavir; LAM, Lamivudine; PEG IFN, Peginterferon; RBV, Ribavirin; TBV, Telbivudine; TAF, Tenofovir alafenamide; TDF, Tenofovir-DF. ADV ETV LAM PEG PEG RBV TBV TAF TDF ADV ETV LAM PEG PEG RBV TBV TAF TDF IFN IFN IFN IFN alfa-2a alfa-2b alfa-2a alfa-2b Anaesthetics & Muscle Relaxants Antibacterials (continued) Bupivacaine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Cloxacillin ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Cisatracurium ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Dapsone ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Isoflurane ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Delamanid ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Ketamine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Ertapenem ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Nitrous oxide ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Erythromycin ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Propofol ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Ethambutol ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Thiopental ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Flucloxacillin ◆ ◆ ◆ ◆ ◆ ◆ Tizanidine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Gentamicin ◆ ◆ ◆ ◆ ◆ ◆ Analgesics Imipenem ◆ ◆ ◆ ◆ ◆ ◆ ◆ Aceclofenac ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Isoniazid ◆ ◆ ◆ ◆ ◆ ◆ Alfentanil ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Levofloxacin ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Aspirin ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Linezolid ◆ ◆ ◆ ◆ ◆ ◆ Buprenorphine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Lymecycline ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Celecoxib ◆ ◆ ◆ ◆ ◆ ◆ ◆ Meropenem ◆ ◆ ◆ ◆ ◆ ◆ Codeine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ for distribution. for Methenamine ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Dexketoprofen ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Metronidazole ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Dextropropoxyphene ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ Moxifloxacin ◆ ◆ ◆ -

HPLC Method Development and Validation: for Simultaneous Determination of Flibanserin and Caffeine
Open Access Journal of Pharmaceutical Research ISSN: 2574-7797 MEDWIN PUBLISHERS Committed to Create Value for Researchers HPLC Method Development and Validation: For Simultaneous Determination of Flibanserin and Caffeine Sharma P1, Dahiya M2, Wakode S2* and Rani R2 Research Article 1Department of Quality assurance, Delhi Institute of Pharmaceutical sciences and Research, Volume 4 Issue 3 India Received Date: August 01, 2020 2Pharmaceutical Chemistry Division, Indian Pharmacopoeia Commission, India Published Date: August 24, 2020 DOI: 10.23880/oajpr-16000213 *Corresponding author: Prof Sharad Wakode, Delhi Institute of Pharmaceutical Sciences and Research Sector-III, MB Road, Pushp Vihar, New Delhi, India-110017, Tel: +91-9891008594; Email: [email protected] Abstract A simple and rapid HPLC method is developed and validated for the simultaneous determination of Flibanserin and Caffeine. ammonium acetate buffer (pH 3) and ACN as the mobile phase, a C18 column and wavelength set at 254nm. The retention time This is the first single reported method for these two drugs. The good separation was achieved by HPLC technique using 0.1% for Caffeine and Flibanserin is 2.0 min and 4.9 min respectively. The method was validated as per ICH guidelines for linearity, precision, accuracy, LOD, LOQ, robustness and solution stability. The method shows good accuracy and precision with RSD mL. value of less than 2%. The Flibanserin shows good linearity in range 50.0µg-150.0µg/mL and Caffeine in range 10.0µg-30.0µg/ Keywords: HPLC, Flibanserin, Caffeine, Validation, HSDD Introduction treatment in women due to certain safety concern. Flibanserin Flibanserin is 1-[2-(1,3-dihydro-2-oxobenzimidazol-1- molecular mass is 390.4g/mol (C20H21F3N4O) and freely yl)ethyl]piperazine approved by US FDA in the year 2015 for solubleshows highin ACN plasma sparingly protein soluble binding in methanol(98% to albumin).and in acidic Its the treatment of hypoactive sexual desire disorder(HSDD) pH however, it is slightly soluble in water, ethanol and formic in women. -
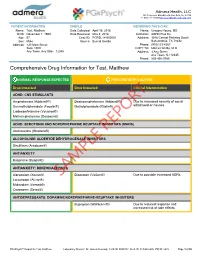
Sample Report
Admera Health, LLC 126 Corporate Blvd ā South Plainfield, NJ 07080 +1-908-222-0533ā[email protected] PATIENT INFORMATION SAMPLE REFERRING PHYSICIAN Name: Test, Matthew Date Collected: April 16, 2018 Name: Gregory House, MD DOB: November 1, 1960 Date Received: May 4, 2018 Institution: ADHD Plus Inc Age: 57 Case ID: PGPSL18-000001 Address: 1046 Central Parkway South Sex: Male Source: Buccal Swabs San Antonio, TX 78232 Address: 123 Main Street Phone: (910)123-4567 Suite 1000 COPY TO: Marcus Welby, M.D. Any Town, Any State 12345 Address: 1 Any Street Any Town, NJ 12345 Phone: 555-456-5768 Comprehensive Drug Information for Test, Matthew NORMAL RESPONSE EXPECTED PROCEED WITH CAUTION Drug Impacted Drug Impacted Clinical Interpretation ADHD: CNS STIMULANTS $PSKHWDPLQH $GGHUDOO 'H[WURDPSKHWDPLQH $GGHUDOO Due to increased severity of social 'H[PHWK\OSKHQLGDWH )RFDOLQ 0HWK\OSKHQLGDWH 5LWDOLQ withdrawal or nausea /LVGH[DPIHWDPLQH 9\YDQVH 0HWKDPSKHWDPLQH 'HVR[\Q ADHD: SEROTONIN AND NOREPINEPHRINE REUPTAKE INHIBITORS (SNRIS) $WRPR[HWLQH 6WUDWWHUD REPORT ALCOHOLISM: ALDEHYDE DEHYDROGENASE INHIBITORS 'LVXOILUDP $QWDEXVH ANTIANXIETY %XVSLURQH %XVSDU ANTIANXIETY: BENZODIAZEPINES $OSUD]RODP ;DQD[ SAMPLE'LD]HSDP 9DOLXP Due to possible increased ADRs /RUD]HSDP $WLYDQ 0LGD]RODP 9HUVHG 2[D]HSDP 6HUD[ ANTIDEPRESSANTS: DOPAMINE/NOREPINEPHRINE-REUPTAKE INHIBITORS %XSURSLRQ :HOOEXWULQ Due to reduced response and increased risk of side effects PGxPsych™ Report for Test, Matthew Laboratory Director: Dr. James Dermody CLIS ID: 0005783 CLIA ID: 31D2038676 -

PHARMACEUTICAL APPENDIX to the HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev
Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM GADOCOLETICUM 280776-87-6 ABAFUNGIN 129639-79-8 ACIDUM LIDADRONICUM 63132-38-7 ABAMECTIN 65195-55-3 ACIDUM SALCAPROZICUM 183990-46-7 ABANOQUIL 90402-40-7 ACIDUM SALCLOBUZICUM 387825-03-8 ABAPERIDONUM 183849-43-6 ACIFRAN 72420-38-3 ABARELIX 183552-38-7 ACIPIMOX 51037-30-0 ABATACEPTUM 332348-12-6 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABETIMUSUM 167362-48-3 ACIVICIN 42228-92-2 ABIRATERONE 154229-19-3 ACLANTATE 39633-62-0 ABITESARTAN 137882-98-5 ACLARUBICIN 57576-44-0 ABLUKAST 96566-25-5 ACLATONIUM NAPADISILATE 55077-30-0 ABRINEURINUM 178535-93-8 ACODAZOLE 79152-85-5 ABUNIDAZOLE 91017-58-2 ACOLBIFENUM 182167-02-8 ACADESINE 2627-69-2 ACONIAZIDE 13410-86-1 ACAMPROSATE