Moderna 2021 Proxy Statement
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Prostate Cancer in Focus
PROSTATE CANCER IN FOCUS Current Developments in the Management of Prostate Cancer Section Editor: Andrew J. Armstrong, MD Prostate Cancer Prostate PSMA-Targeted Therapy in Prostate Cancer Scott T. Tagawa, MD, MS Professor of Medicine and Urology Weill Cornell Medicine New York, New York H&O What is prostate-specific membrane agent such as a monoclonal antibody might be able to antigen (PSMA), and what makes it a good target target a tumor without binding to other PSMA-positive for the treatment of prostate cancer? sites owing to its large size and physical inability to reach luminal sites of expression that are separated from the ST PSMA is a cell surface antigen that is expressed to vasculature by tight junctions. a limited degree on certain normal cells in the body but generally is highly overexpressed in the setting of prostate H&O How is PSMA positivity established? cancer. Normal cells that express PSMA are located in the prostate, salivary, and lacrimal glands; the proximal part ST Imaging and biopsy are the main ways to establish of the small intestine; the proximal renal tubules; and PSMA positivity. Multiple imaging modalities are avail- some ganglia. In the setting of prostate cancer, PSMA able worldwide. The agent indium In 111 capromab expression generally increases along with the cancer grade, pendetide (ProstaScint) has been approved for years in and it also increases following hormonal therapy. In addi- the United States, and the US Food and Drug Adminis- tion, PSMA expression tends to be higher in metastatic tration (FDA) approved the use of gallium Ga 68 PSMA- sites than in primary tumors. -

Alkermes Public Limited Company
ALKERMES PUBLIC LIMITED COMPANY Directors’ Report and Consolidated Financial Statements For the Year Ended March 31, 2013 ALKERMES PLC Table of Contents Page Directors’ Report .......................................................... 2 Statement of Directors’ Responsibilities .......................................... 56 Independent Auditors’ Report—Group ........................................... 57 Consolidated Profit and Loss Account ........................................... 59 Consolidated Statement of Comprehensive Income (Loss) ............................. 60 Consolidated Balance Sheet ................................................... 61 Consolidated Statement of Cash Flows ........................................... 63 Consolidated Reconciliation of Shareholders’ Funds ................................. 62 Notes to the Consolidated Financial Statements .................................... 64 Independent Auditors’ Report—Company ......................................... 111 Company Balance Sheet ..................................................... 113 Notes to the Company Financial Statements ....................................... 114 1 DIRECTORS’ REPORT For the Year Ended March 31, 2013 The directors present their report and audited consolidated financial statements for the fiscal year ended March 31, 2013. The directors have elected to prepare the consolidated financial statements in accordance with section 1 of the Companies (Miscellaneous Provisions) Act, 2009, which provides that a true and fair view of the state of -

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -

In Re: Alkermes Securities Litigation 03-CV-12091-Consolidated
Case 1:03-cv-12091-RCL Document 39 Filed 07/12/2004 Page 1 of 49 UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS In re ALKERMES SECURITIES ) Master Docket No. 03-CV-12091-RCL LITIGATION ) ) CLASS ACTION This Document Relates To: ) ) ALL ACTIONS. ) ) ) CONSOLIDATED COMPLAINT FOR VIOLATION OF THE FEDERAL SECURITIES LAWS Case 1:03-cv-12091-RCL Document 39 Filed 07/12/2004 Page 2 of 49 SUMMARY AND OVERVIEW 1. This is a securities class action on behalf of all purchasers of the common stock of Alkermes, Inc. (“Alkermes” or the “Company”) between April 22, 1999 and July 1, 2002 (the “Class Period”), against Alkermes and certain of its officers and directors for violations of the Securities Exchange Act of 1934 (the “Exchange Act”). 2. Alkermes is a biopharmaceutical company focused on the development of controlled- release drug delivery technologies and their application to existing or new drug therapies. Among the drug delivery technologies defendants seek to develop are sustained-release systems based on biodegradable polymeric microspheres, including those based on Medisorb polymers. 3. In 1996, Alkermes entered into an agreement with JPI Pharmaceutical International (“Janssen”), an affiliate of pharmaceutical giant Johnson & Johnson Pharmaceutical Research & Development, L.L.C. (“Johnson & Johnson”), to develop an injectable form of the schizophrenic drug Risperdal, based upon the Medisorb polymer technology, called Risperdal Consta. Janssen has marketed the oral form of Risperdal since 1993, with sales of $2.3 billion in 2003. According to a December 12, 2001 report by analysts Thomas Weisel Partners, LLC (“Thomas Weisel”), during the Class Period, oral Risperdal was the most prescribed drug in the $5.3 billion atypical antipsychotic market. -

Explanation of Conflict of Interest Disclosure Parts: Part One: All
Explanation of Conflict of Interest Disclosure Parts: Part One: All Financial Involvement with a pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical companies doing business with or proposing to do business with ACNP over past 2 years (Jan. 2011-Present) Part Two: Income Sources & Equity of $10,000 or greater Part Three: Financial Involvement with a pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical products or companies doing business with or proposing to do business with ACNP which constitutes more than 5% of personal income (Jan. 2011-Present): Part Four: Grants from pharmaceutical or biotechnology company, a company providing clinical assessment, scientific, or medical products directly, or indirectly through a foundation, university, or any other organization (Jan. 2011-Present) Part Five: My primary employer is a pharmaceutical/biotech/medical device company. 2012 Program Committee Disclosures Anissa Abi-Dargham: Part 1: Pfizer; Otsuka; Takeda; Sunovion; Shire; Roche; Pierre Favre; Part 2: Pierre Favre William Carlezon: Part 1: Referring to 2010-2011: Scientific Advisory Board, Myneurolab.com; Consultant, Concert Pharmaceuticals; Consultant, Lantheus Medical Imaging; Consultant, Transcept Pharmaceuticals, (Spouse) Senior Scientist, EMD Serono; Part 2: (Spouse) Senior Scientist, EMD Serono, Part 3: (Spouse) Senior Scientist, EMD Serono Cameron Carter: Part1: GlaxoSmithKline research; Part 4: GlaxoSmithKline Karl Deisseroth: Part -

Objectives & Accreditation
Objectives & Accreditation ACTIVITY FORMAT Live TARGET AUDIENCE This activity is designed to meet the needs of physicians, physician assistants, pharmacists, registered nurses, nurse practitioners, advance practice registered nurses, and registered dietitians with an interest in lipid management. TYPE OF ACTIVITY Live Activity; Application/ Knowledge EDUCATIONAL OBJECTIVES At the conclusion of this activity, Registered Nurses and Nurse Practitioners should be able to provide appropriate care and counsel for patients and their families. At the conclusion of this activity, all participants should be able to: Session I: The Impact of Genetics in Lipidology? Apply insight from human population genetics studies to identify novel therapeutic targets for a better understanding for atherosclerotic cardiovascular disease. Describe the current and future technologies available for genetic screen includinghow to apply them in clinical practice both for diagnosis and risk assessment Discuss experimental techniques in development for genetic manipulation to target inherited lipid disorders Keynote I Discuss ApoC3 as both a risk factor for ASCVD and as potential for therapeutic manipulation Session II- Focus on Lipoprotein (a) Discuss the epidemiology of Lipoprotein(a) (LP(a)) for both thrombosis and ASCVD Discuss the pathophysiology of LP(a) and how this impacts increased risk of ASCVD and thrombosis Discuss the status of current and emerging treatment options targeting LP(a) Clinical Lipidology Track- Critically appraise emerging research -

Pioneering New Markets Changing the Standard of Care
ANNUAL 2020 REPORT Pioneering New Markets Changing the Standard of Care UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2020 □ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 000-19125 Ionis Pharmaceuticals, Inc. (Exact name of Registrant as specified in its charter) Delaware 33-0336973 (State or other jurisdiction of (IRS Employer incorporation or organization) Identification No.) 2855 Gazelle Court, Carlsbad, CA 92010 (Address of Principal Executive Offices) (Zip Code) 760-931-9200 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading symbol Name of each exchange on which registered Common Stock, $.001 Par Value ‘‘IONS’’ The Nasdaq Stock Market LLC Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No □ Indicate by check if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes □ No ☒ Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Interim Recommendations for Use of the Moderna Mrna-1273 Vaccine Against COVID-19
Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 Interim guidance First issued 25 January 2021 Updated 15 June 2021 Background This interim guidance has been developed on the basis of the advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) at its extraordinary meeting on 21 January 2021 (1) and updated during its extraordinary meeting on 27 May 2021(2). Declarations of interests were collected from all external contributors and assessed for any conflicts of interest. Summaries of the reported interests can be found on the SAGE meeting website and SAGE Working Group website. The guidance is based on the evidence summarised in the Background document on the Moderna mRNA-1273 vaccine against COVID-19 (3) and the background paper on COVID-19 disease and vaccines (4). Annexes which include GRADE and evidence-to-recommendations (ETR) tables have also been updated to reflect the updated recommendations. All referenced documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic- advisory-group-of-experts-on-immunization/covid-19-materials. These interim recommendations refer to the mRNA-1273 vaccine, manufactured by Moderna. The vaccine is also known as COVID-19 Vaccine Moderna. In the subsequent text the vaccine will be referred to as mRNA-1273. On 30 April 2021, mRNA-1273 was granted WHO’s Emergency Use Listing (EUL). Methods SAGE applies the principles of evidence-based medicine and has set in place a thorough methodological process for issuing and updating recommendations (5). A detailed description of the methodological processes as they apply to COVID-19 vaccines can be found in the SAGE evidence framework for COVID-19 vaccines (6). -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -
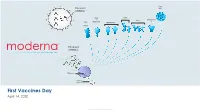
Moderna Vaccine Day Presentation
Zika Encoded VLP mRNA(s) RSV CMV + EBV SARS-CoV- RSV-ped 2 Flu hMPV/PIV3 H7N9 Encoded mRNA(s) Ribosome Protein chain(s) First Vaccines Day April 14, 2020 © 2020 M oderna Therapeutics Forward-looking statements and disclaimers This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended including, but not limited to, statements concerning; the impact of the SARS-CoV-2 pandemic on the Company’s clinical trials and operations; the timing and finalization of a dose-confirmation Phase 2 study and planning for a pivotal Phase 3 study for mRNA-1647; the status and outcome of the Phase 1 clinical trial for mRNA-1273 being conducted by NIH; the next steps and ultimate commercial plan for mRNA-1273; the size of the potential market opportunity for mRNA-1273; the size of the potential commercial market for novel vaccines produced by Moderna or others; the potential peak sales for the Company’s wholly-owned vaccines; the probability of success of the Company’s vaccines individually and as a portfolio; and the ability of the Company to accelerate the research and development timeline for any individual product or the platform as a whole. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Eli Lilly and Company
Merrimack College Merrimack ScholarWorks Honors Senior Capstone Projects Honors Program Spring 2021 Eli Lilly and Company Alyssa Ardai Follow this and additional works at: https://scholarworks.merrimack.edu/honors_capstones Part of the Business Commons Ardai 1 Written Assignment #4 Eli Lilly and Company Alyssa Ardai Bus 4402W: Strategic Analysis and Decision Making Professor Herrmann April 30, 2021 Ardai 2 Abstract Eli Lilly and Company is a pharmaceutical company that has the goal of creating new products. Eli Lilly’s products are seen in hospitals and pharmacies around the US, with the hopes of growing internationally. By having a large number of drugs in their pipeline, they can be a key player in improving multiple types of illnesses as well as help aid the aging population. The healthcare sector is always one that is high-performing. Ardai 3 Eli Lilly and Company is positioned as a pharmaceutical company, with the goal of creating high-quality medicine for every need or to take a preexisting medicine and make it better. For the past 140 years, they have been creating medicine for various causes, but are now focusing their efforts on antibody treatments for the COVID-19 pandemic, as well as different cancers and autoimmune diseases. Everything that the company does should follow its core values: integrity, excellence, and respect for people (Eli Lilly and Company - A). Eli Lilly is found in the US, Japan, Europe, and hopes to expand to the rest of the world soon. They are broken down into Endocrinology, Oncology, Immunology, Neuroscience, & Other. The company has increased revenues from $23,832.8 - $25,925.3, a consistent tax rate of 15%, expenses increasing $300k a year, accounts receivable, or a/r, decreasing as they pay less in patents and drugs go generic, consistent inventory of $120k - $140k, showing they do not keep a lot on hand, a weighted average cost of capital, or WACC, of 5.9% showing it does not hold a lot of debt, and a long term growth rate of 2.3%, showing a healthy but stable growth (Bloomberg LP, CapitalIQ 2021).