The Role of Canopy Gaps in Maintaining Biodiversity of Plants and Soil Macrofauna in the Forests of the Northwestern Caucasus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Hornbeam Carpinus Betulus ‘Fastigiata’
Smart tree selections for communities and landowners European Hornbeam Carpinus betulus ‘Fastigiata’ Height: 35’ Spread: 25’ Site characteristics: Sun to partial shade; prefers moist, well-drained soils Zone: 5a - 7a Wet/dry: Tolerates drought, heavy soil and alkaline soils Native range: Europe, Western Asia Salt: Sensitive pH: 5.0 - 8.2 Shape: Narrow when young, becoming oval Other: Narrow branching angles Additional: Transplant in spring, somewhat slow to establish from bare root Pests: Some occasional dieback Bert Cregg, MSU Bert Cregg, Bert Cregg, MSU Bert Cregg, Bugwood.org Hungary, of West Univ. Norbert Frank, Content development: Dana Ellison, Tree form illustrations: Marlene Cameron. Smart tree selections for communities and landowners Bert Cregg and Robert Schutzki, Michigan State University, Departments of Horticulture and Forestry A smart urban or community landscape has a diverse combination of trees. The devastation caused by exotic pests such as Dutch elm disease, chestnut blight and emerald ash borer has taught us the importance of species diversity in our landscapes. Exotic invasive pests can devastate existing trees because many of these species may not have evolved resistance mechanisms in their native environments. In the recent case of emerald ash borer, white ash and green ash were not resistant to the pest and some communities in Michigan lost up to 20 percent of their tree cover. To promote diverse use of trees by homeowners, landscapers and urban foresters, Michigan State University Extension offers a series of tip sheets for smart urban and community tree selection. In these tip sheets, we suggest trees that should be considered in situations where an ash tree may have been planted in the past. -

Charbrook Nursery
charbrook nursery Plant List Spring-Summer-Autumn 2020 Name Size Wholesale Red Maple 1.5"-2" $200.00 Acer rubrum `Brandywine`, `Frank Jr.`, `Franksred` TM, `Sun Valley` 2"-2.5" $260.00 Single-stem 2.5"-3" $380.00 3"-3.5" $500.00 3.5"-4" $670.00 4"-4.5" $860.00 Sugar Maple 1.5"-2" $250.00 Acer saccharum `Fall Fiesta`, Flashfire 2"-2.5" $300.00 2.5"-3" $380.00 Autumn Blaze Maple 2.5"-3" $360.00 Acer rubrum 'Autumn Blaze' x 'Freemanii' 3"-3.5" $470.00 3.5"-4" $640.00 4"-4.5" $850.00 Shadblow Serviceberry 4'-5' $195.00 Amelanchier x grandiflora 'Autumn Brillance' 5'-6' $240.00 Multi-stem 6'-7' $285.00 7'-8' $420.00 8'-10' $630.00 10'-12' $855.00 Yellow Birch 1"-1.5" $180.00 Betula alleghaniensis 1.5"-2" $250.00 2"-2.5" $350.00 2.5"-3" $460.00 Sweet Birch 1.5"-2" $210.00 Betula lenta 2"-2.5" $300.00 2.5"-3" $400.00 Paper Birch 1.5"-2" $210.00 Betula papyrifera 2"-2.5" $300.00 Betula papyrifera `Varen` 2.5"-3" $400.00 Charbrook Nursery 71 Gates Road Princeton, MA 01541 Page 1 Franz Fontaine Hornbeam 2"-2.5" $320.00 Carpinus betulus `Franz Fontaine` 2.5"-3" $460.00 3"-3.5" $620.00 American Hornbeam 2"-2.5" $300.00 Carpinus caroliniana 2.5"-3" $390.00 3"-3.5" $510.00 Northern Catalpa 2.5"-3" $360.00 Catalpa speciosa 3"-3.5" $470.00 3.5"-4" $690.00 4"-4.5" $900.00 Giant Dogwood 2"-2.5" $280.00 Cornus controversa `June Snow` 2.5"-3" $350.00 Tamarack 10'-12' $400.00 Larix laricina 12'-14' $530.00 (Spring dig only) 14'-16' $710.00 Crab Apple 1.5"-2" $210.00 Malus x `Donald Wyman` , `Schmidtcutleaf` TM, `Snowdrift` 2"-2.5" $260.00 American Hophornbeam -

Hornbeam Carpinus Betulus
34 W I N G E D S E E D S W I N G E D S E E D S 35 Sycamore Acer pseudoplatanus Hornbeam Carpinus betulus Leaves are large and five-lobed, with ornbeams are native trees found largely in south-eastern his introduced species grows in a wide dark green upper range of habitats and soil types. Sycamore sides.The seeds HEngland, with scattered trees in other parts of the country. T come in pairs, that They tolerate a wide range of soils, including sands, gravels and is an excellent coloniser and is often consid- are joined together ered a problem species as, in certain habitats, at an angle. heavy clay, but grow best on damp, fertile soils. Hornbeams including woodland, it can become the domi- produce excellent autumn colours, retaining their leaves throughout much of the winter. nant species. Large tree (5:10:25) Sycamore wood is light in colour, strong and One of the hardest and toughest woods in hard, and is used for kitchen table-tops, floor- Britain, the name hornbeam derives ing, veneers and toys. from the fact that the wood is as This species supports a limited number hard as horn. It was used for of insect species, which includes large cattle yokes, waterwheels and numbers of aphids. In consequence, butchers’ chopping blocks. The migrating warblers can often be found timber also makes excellent fire- feeding in sycamores in the autumn. The wood. tree also supports good lichen growth, Hornbeams are valu- particularly in the west of Britain. able to wildlife, pro- ducing nutlets Seed Guide: Collect the fruits from the which are eaten tree in autumn when they turn by hawfinches brown. -

SP532 Trees to Plant in Containers Or Wells
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Forestry, Trees, and Timber UT Extension Publications 3-1999 SP532 Trees to Plant In Containers or Wells The University of Tennessee Agricultural Extension Service Follow this and additional works at: https://trace.tennessee.edu/utk_agexfores Part of the Plant Sciences Commons Recommended Citation "SP532 Trees to Plant In Containers or Wells," The University of Tennessee Agricultural Extension Service, SP 532-15M-3/99 R12-4910-17-001-00, https://trace.tennessee.edu/utk_agexfores/54 The publications in this collection represent the historical publishing record of the UT Agricultural Experiment Station and do not necessarily reflect current scientific knowledge or ecommendations.r Current information about UT Ag Research can be found at the UT Ag Research website. This Trees for Tennessee Landscapes - Choosing the Right Tree is brought to you for free and open access by the UT Extension Publications at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Forestry, Trees, and Timber by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Agricultural Extension Service The University of Tennessee SP 532 Trees to Plant In Containers or Wells Donna C. Fare Wayne K. Clatterbuck Research Horticulturist Assistant Professor USDA-ARS Forestry, Wildlife US National Arboretum & Fisheries Donna C. Fare C. Donna Donna C. Fare C. Donna Pleasing example of a group of trees growing in containers. An in-ground tree planter with a magnolia tree. Landscaping in a small area is challenging, but popular. An important aspect of container or well planting is the Planting trees in small areas can limit root and shoot devel- soil or growing medium. -

The Relationship Among Infection Intensity of Viscum Album with Some Ecological Parameters of Host Trees
Int. J. Environ. Res., 1(2): 143-149, Spring 2007 ISSN: 1735-6865 The Relationship among Infection Intensity of Viscum album with some Ecological Parameters of Host Trees Kartoolinejad, D.1*, Hosseini, S. M.1, Mirnia, S. K.2, Akbarinia, M.1 and Shayanmehr, F.1 1Natural Resources Faculty, Tarbiat Modares University, Tehran, Iran 2Agriculture Faculty, Tarbiat Modares University, Tehran, Iran Received 20 March 2006; Revised 12 Dec 2006; Accepted 10 Jan 2007 ABSTRACT: We investigated the relations among infection intensity of European mistletoe (Viscum album L.) with host tree features in Nour Forest Park, located in Caspian Forests in North of Iran. The number of 30 circular plots with an area of 0.1 ha were sampled in all places have an aggregation of infested trees. Parameters including DBH, height, distance to stand edge, distance to conspecific tree, bark diameter and the number of adult mistletoe per tree for all infected individuals were recorded. Results showed that the mistletoe abundance and infection intensity in Parrotia persica was more than the other host species and also, have positive significant relation with DBH, distance to conspecific and locating in the stand edge, but no significant relation observed about height of host trees. Results of this study suggest that individual differences among host trees (specially DBH) play an important role in explaining local abundance and distribution of mistletoe plants. Key words: Infection intensity, European mistletoe, Host trees, Caspian forests, Conspecific tree *Corresponding author: [email protected] INTRODUCTION Mistletoes are a polyphyletic and diverse real root, parasitize the stems of dicotyledonous group of flowering plants comprising over 1306 trees and shrubs by means of parenchyma organs species from a broad range of habitats across all named haustoria, developing from the radicle of continents except Antarctica. -
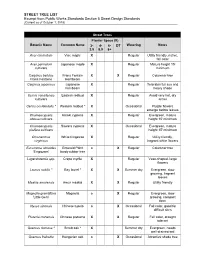
Approved Street Tree and Shrub List, from Public Works Standards
STREET TREE LIST Excerpt from Public Works Standards Section 5 Street Design Standards (Current as of October 1, 2019) Street Trees Planter Space (ft) Botanic Name Common Name 3- 4- 6- DT Watering Notes 3.9 5.9 8+ Acer circinatum Vine maple X Regular Utility friendly, native, fall color Acer palmatum Japanese maple X Regular Mature height 15' cultivars minimum Carpinus betulus Frans Fontain X X Regular Columnar tree Frans Fontaine Hornbeam Carpinus japonicus Japanese X Regular Tolerates full sun and hornbeam heavy shade Cercis canadensis Eastern redbud X Regular Avoid very hot, dry cultivars areas Cercis occidentalis * Western redbud * X Occasional Purple flowers emerge before leaves Chamaecyparis Hinoki cypress X Regular Evergreen, mature obtusa cultivars height 15' minimum Chamaecyparis Sawara cypress X Occasional Evergreen, mature pisifera cultivars height 15' minimum Chionanthus White fringetree X Regular Utility friendly, virginicus fragrant white flowers Eucommia ulmoides Emerald Point o X Regular Columnar tree 'Empozam' hardy rubber tree Lagerstroemia spp. Crepe myrtle X Regular Vase-shaped, large flowers Laurus nobilis * Bay laurel * X X Summer dry Evergreen, slow growing, fragrant leaves Maakia amurensis Amur maakia X X Regular Utility friendly Magnolia grandiflora Magnolia o X Regular Evergreen, slow 'Little Gem' growing, compact form Nyssa sinensis Chinese tupelo o X Occasional Fall color, good for difficult sites Pistacia chinensis Chinese pistache X X Regular Fall color, drought tolerant Quercus dumosa * Scrub oak * X Summer -

Carpinus Betulus - European Hornbeam (Betulaceae) ------Carpinus Betulus Is a Columnar to Teardrop-Shaped Tree
Carpinus betulus - European Hornbeam (Betulaceae) ------------------------------------------------------------------------------------------ Carpinus betulus is a columnar to teardrop-shaped tree. Twigs European Hornbeam is noted for fine and dense texture, -olive-brown and lenticeled, with ornamental winter ornamental winter bark and buds, dense summer foliage, buds that are long and partially curving around the twigs pendulous spring catkins, and unusual autumn fruits. -the twigs are similar to those of the European Beech, but the latter has winter buds that extend straight out of FEATURES the stem at a 45 degree angle. Form Trunk -medium-sized deciduous tree; the -smooth and steel gray, but having a muscled character rarely available species form maturing to its appearance at 40' tall x 30' wide, with the common cultivars more compact; USAGE species form an upright oval growth Function habit in youth, quickly becoming a -specimen or focal point tree of great symmetrical and spreading oval (low-branched teardrop architectural value; can also be an effective year-round shape) with maturity screen or tall, wide hedge when used in rows -medium growth rate Culture Texture -full sun to partial sun; prefers a well-drained soil but is -fine texture in foliage and when bare; thick density in adaptable to various soils and soil pHs; if transplanted foliage and when bare, with many ascending twigs and in autumn, use amended soil, fertilize, mulch liberally, branches forming a thick canopy even in winter and avoid winter salt spray Assets -cultivars -

IHCA Recommended Plant List
Residential Architectural Review Committee Recommended Plant List Plant Materials The following plant materials are intended to guide tree and shrub ADDITIONS to residential landscapes at Issaquah Highlands. Lot sizes, shade, wind and other factors place size and growth constraints on plants, especially trees, which are suitable for addition to existing landscapes. Other plant materials may be considered that have these characteristics and similar maintenance requirements. Additional species and varieties may be selected if authorized by the Issaquah Highlands Architectural Review Committee. This list is not exhaustive but does cover most of the “good doers” for Issaquah Highlands. Our microclimate is colder and harsher than those closer to Puget Sound. Plants not listed should be used with caution if their performance has not been observed at Issaquah Highlands. * Drought-tolerant plant ** Requires well-drained soil DECIDUOUS TREES: Small • Acer circinatum – Vine Maple • Acer griseum – Paperbark Maple • *Acer ginnala – Amur Maple • Oxydendrum arboreum – Sourwood • Acer palmation – Japanese Maple • *Prunus cerasifera var. – Purple Leaf Plum varieties • Amelanchier var. – Serviceberry varieties • Styrax japonicus – Japanese Snowbell • Cornus species, esp. kousa Medium • Acer rufinerve – Redvein Maple • Cornus florida (flowering dogwood) • *Acer pseudoplatanus – Sycamore Maple • Acer palmatum (Japanese maple, many) • • *Carpinus betulus – European Hornbeam Stewartia species (several) • *Parrotia persica – Persian Parrotia Columnar Narrow -

Carpinus L. Hornbeam Or Ironwood
C Betulaceae—Birch family Carpinus L. hornbeam or ironwood Paula M. Pijut Dr. Pijut is a plant physiologist at the USDA Forest Service’s North Central Research Station, Hardwood Tree Improvement and Regeneration Center,West Lafayette, Indiana Growth habit, occurrence, and use. The hornbeam Appalachian Mountains and northern interior regions to the genus—Carpinus L.—includes about 35 species of decidu- West (Furlow 1987b). The Latin American C. tropicalis is ous, monoecious, small to large trees, that are native to the divided into subsp. tropicalis of the highlands of southern Northern Hemisphere from Europe to eastern Asia, south to Mexico and north Central America and subsp. mexicana of the Himalayas, and in North and Central America (Furlow the mountains in northeastern Mexico and the trans-Mexican 1990; Hillier 1991; Krüssmann 1984; LHBH 1976; Suszka volcanic belt (Furlow 1987b). and others 1996). Five species are considered here (table The wood of hornbeams is extremely hard—hence the 1). Hornbeams occur mainly as understory trees in rich, common name “ironwood”—and is used for making tool moist soils on bottomlands and on protected slopes handles and mallet heads. It is also used to produce the (Metzger 1990; Rudolf and Phipps 1974). European horn- high-quality charcoal used in gunpowder manufacture beam is an important forest tree species throughout Europe (Bugala 1993; Furlow 1990). Species of ornamental interest (Furlow 1990). In Mexico and Central America, Carpinus in the United States are listed in table 1. Most of the infor- tropicalis (J.D. Sm.) Lundell forms a dominant canopy mation presented in this chapter deals with European and component (Furlow 1990). -

Morphometric and Physiological Analysis of Fagus Sylvatica and Carpinus Betulus Leaves
Research Journal of Agricultural Science, 52 (3), 2020 MORPHOMETRIC AND PHYSIOLOGICAL ANALYSIS OF FAGUS SYLVATICA AND CARPINUS BETULUS LEAVES A.G. KOLOZSVARI1, Adina-Daniela DATCU1,2, F. SALA1 1Banat’s University of Agricultural Sciences and Veterinary Medicine „King Michael I of Romania” from Timișoara, Soil Science and Plant Nutrition 2West University of Timișoara, Faculty of Chemistry, Biology, Geography, Biology-Chemistry Department *Corresponding author: [email protected] Abstract. This study purpose was to assess some morphometric and physiological indices at two woody plant species, European beech (Fagus sylvatica L.) and common hornbeam (Carpinus betulus L.), to describe plant behavior in different forest zones, at different altitudes and light intensities. The samples were collected from a beech forest located in western part of Romania, at an average altitude of 230 a.s.l., in October 2020. Leaves that were intact and healthy were selected. These were scanned and then taken into plant physiology lab for dry weight determination. The analyzed indices were leaves lengths, perimeters and areas with a specific software. Dry weight of each sample was determined using an analytical balance, after the completion of drying process in an oven, at 100 ˚C. After this, specific leaf weight (SLW) was obtained. Regarding the results, in general, beech leaves were longer and with a higher perimeter than common hornbeam ones. The highest values of this indices were obtained for beech samples collected from the forest. Leaf area was higher for beech leaves, when compared with hornbeam leaves. Between gravimetric indices tested in this study, dry weight behavior was similarly with leaf areas one. -

Scolytus Intricatus (Ratzeburg) Coleoptera: Curculionidae European Oak Bark Beetle
Scolytus intricatus (Ratzeburg) Coleoptera: Curculionidae European Oak Bark Beetle CAPS-Approved Survey Host(s) Method Major/Primary hosts Visual Castanea sativa (Sweet chestnut), Corylus colurna, Quercus spp. (Oak), Quercus coccifera (Kermes oak), Quercus dalechampii (Dalechamp’s oak), Quercus ilex (Holly oak), Quercus petraea (Durmast oak), Quercus robur (Common oak) Other hosts Aesculus spp. (Buckeye), Aesculus hippocastanum (Horse chestnut), Alnus glutinosa (European alder), Betula spp. (Birch), Betula celtiberica (Iberian white birch), Betula pendula (Common silver birch), Betula pubescens (Downy birch), Betula verrucosa (European white birch), Carpinus spp. (Hornbeam), Carpinus betulus (European hornbeam), Castanea spp. (Chestnut), Castanea vesca (Sweet chestnut), Corylus spp. (Hazelnut), Fagus spp. (Beech), Fagus moesiaca, Fagus orientalis (Oriental beech), Fagus sylvatica (Common beech), Ostrya spp. (Hop-hornbeam), Ostrya carpinifolia (Hop-hornbeam), Parrotia persica (Persian parrotia), Populus spp. (Poplars), Populus alba (White poplar), Populus tremula (European aspen), Quercus canariensis (Algerian oak), Quercus castaneaefolia (Chestnut-leaf oak), Quercus cerris (European turkey oak), Quercus frainetto (Italian oak), Quercus hartwissiana, 1 Quercus lusitanica (Lusitanian oak), Quercus polycarpa, Quercus prinus var. tomentosa, Quercus pubescens (Downy oak), Quercus pyrenaica (Pyrenean oak), Quercus rubra (Northern red oak), Quercus virgiliana (Italian oak), Salix spp. (Willow), Sorbus spp. (Mountain Ash), Tilia cordata (Small-leaf lime), Ulmus spp. (Elms), Ulmus carpinifolia (English elm), Ulmus laevis (European white elm), Zelkova carpinifolia (Caucasian zelkova) (Davis et al., 2006; Kimoto and Duthie-Holt, 2006; CABI, 2008) Reason for Inclusion in Manual Scolytus intricatus was added to the manual in 2010. Scolytus intricatus is a target pest on the FY2011 AHP Prioritized Pest List. Pest Description Eggs: Eggs are a pearly white color and oval 1 with a length of 1 mm (< /16 in) (Haack, 2001). -

(Carpinus Turczaninovii) Based on Nuclear Ribosomal ITS Sequence
African Journal of Biotechnology Vol. 10(76), pp. 17435-17442, 30 November, 2011 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.1337 ISSN 1684–5315 © 2011 Academic Journals Full Length Research Paper Phylogeny of Korean Hornbeam ( Carpinus turczaninovii ) based on nuclear ribosomal ITS sequence Sun, Y. L. 1, Wang, D. 1, Lee, H. B.2, Park, W. G. 2, Kwon, O. W. 3 and Hong, S. K.1,4 * 1Department of Bio-Health Technology, Kangwon National University, Chuncheon, Kangwon-Do, 200-701, Korea. 2Department of Forest Resources, Kangwon National University, Chuncheon, Kangwon-Do, 200-701, Korea. 3Korea Forest Seed and Variety Center, Suanbo, Chungju, Chungcheongbuk-Do, 380-941, Korea. 4Division of Biomedical Technology, College of Biomedical Science, Chuncheon, Kangwon-Do, 200-701, Korea. Accepted 25 July, 2011 The genus Carpinus belonging to Coryloideae, Betulaceae, has significant economic and ornamental importance. This study was undertaken with the aim to understand the genetic diversity among eighteen isolates of Carpinus turczaninovii collected from different geographical regions of Korea, using ribosomal RNA (rRNA) internal transcribed spacer (ITS) sequences, to compare the infraspecific- phylogenetic relationships among C. turczaninovii in Korea, and some known Carpinus plants. The size variation of sequenced rRNA ITS regions was not seen, with 215, 162, 222 bp of ITS1 region, 5.8S rRNA gene, ITS2 region, respectively. However, some certain nucleotide variations resulted in genetic diversity. In the genus Carpinus , C. turczaninovii closely genetic with Castanopsis kawakamii , Carpinus orientalis , Carpinus monbeigiana , and Calyptranthes polynenra formed one monophyletic clade, while Carpinus betulus and Carpinus laxiflora, respectively formed one monophyletic clade.