Successional Categorization of European Hemi-Boreal Forest Tree Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Hornbeam Carpinus Betulus ‘Fastigiata’
Smart tree selections for communities and landowners European Hornbeam Carpinus betulus ‘Fastigiata’ Height: 35’ Spread: 25’ Site characteristics: Sun to partial shade; prefers moist, well-drained soils Zone: 5a - 7a Wet/dry: Tolerates drought, heavy soil and alkaline soils Native range: Europe, Western Asia Salt: Sensitive pH: 5.0 - 8.2 Shape: Narrow when young, becoming oval Other: Narrow branching angles Additional: Transplant in spring, somewhat slow to establish from bare root Pests: Some occasional dieback Bert Cregg, MSU Bert Cregg, Bert Cregg, MSU Bert Cregg, Bugwood.org Hungary, of West Univ. Norbert Frank, Content development: Dana Ellison, Tree form illustrations: Marlene Cameron. Smart tree selections for communities and landowners Bert Cregg and Robert Schutzki, Michigan State University, Departments of Horticulture and Forestry A smart urban or community landscape has a diverse combination of trees. The devastation caused by exotic pests such as Dutch elm disease, chestnut blight and emerald ash borer has taught us the importance of species diversity in our landscapes. Exotic invasive pests can devastate existing trees because many of these species may not have evolved resistance mechanisms in their native environments. In the recent case of emerald ash borer, white ash and green ash were not resistant to the pest and some communities in Michigan lost up to 20 percent of their tree cover. To promote diverse use of trees by homeowners, landscapers and urban foresters, Michigan State University Extension offers a series of tip sheets for smart urban and community tree selection. In these tip sheets, we suggest trees that should be considered in situations where an ash tree may have been planted in the past. -

Dynamics and Disturbance in an Old-Growth Forest Remnant In
DYNAMICS AND DISTURBANCE IN AN OLD-GROWTH FOREST REMNANT IN WESTERN OHIO Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Sean Michael Goins UNIVERSITY OF DAYTON Dayton, Ohio August, 2012 DYNAMICS AND DISTURBANCE IN AN OLD-GROWTH FOREST REMNANT IN WESTERN OHIO Name: Goins, Sean Michael APPROVED BY: __________________________________________ Ryan W. McEwan, Ph.D. Committee Chair Assistant Professor __________________________________________ M. Eric Benbow, Ph.D. Committee Member Assistant Professor __________________________________________ Mark G. Nielsen, Ph.D. Committee Member Associate Professor ii ABSTRACT DYNAMICS AND DISTURBANCE IN AN OLD-GROWTH FOREST REMNANT IN WESTERN OHIO Name: Goins, Sean Michael University of Dayton Advisor: Dr. Ryan W. McEwan Forest communities are dynamic through time, reacting to shifts in disturbance and climate regimes. A widespread community shift has been witnessed in many forests of eastern North America wherein oak (Quercus spp.) populations are decreasing while maple (Acer spp.) populations are increasing. Altered fire regimes over the last century are thought to be the primary driver of oak-to-maple community shifts; however, the influence of other non-equilibrium processes on this community shift remains under- explored. Our study sought to determine the community structure and disturbance history of an old-growth forest remnant in an area of western Ohio where fires were historically uncommon. To determine community structure, abundance of woody species was measured within 32 plots at 4 canopy strata and dendrochronology was used to determine the relative age-structure of the forest. -

Charbrook Nursery
charbrook nursery Plant List Spring-Summer-Autumn 2020 Name Size Wholesale Red Maple 1.5"-2" $200.00 Acer rubrum `Brandywine`, `Frank Jr.`, `Franksred` TM, `Sun Valley` 2"-2.5" $260.00 Single-stem 2.5"-3" $380.00 3"-3.5" $500.00 3.5"-4" $670.00 4"-4.5" $860.00 Sugar Maple 1.5"-2" $250.00 Acer saccharum `Fall Fiesta`, Flashfire 2"-2.5" $300.00 2.5"-3" $380.00 Autumn Blaze Maple 2.5"-3" $360.00 Acer rubrum 'Autumn Blaze' x 'Freemanii' 3"-3.5" $470.00 3.5"-4" $640.00 4"-4.5" $850.00 Shadblow Serviceberry 4'-5' $195.00 Amelanchier x grandiflora 'Autumn Brillance' 5'-6' $240.00 Multi-stem 6'-7' $285.00 7'-8' $420.00 8'-10' $630.00 10'-12' $855.00 Yellow Birch 1"-1.5" $180.00 Betula alleghaniensis 1.5"-2" $250.00 2"-2.5" $350.00 2.5"-3" $460.00 Sweet Birch 1.5"-2" $210.00 Betula lenta 2"-2.5" $300.00 2.5"-3" $400.00 Paper Birch 1.5"-2" $210.00 Betula papyrifera 2"-2.5" $300.00 Betula papyrifera `Varen` 2.5"-3" $400.00 Charbrook Nursery 71 Gates Road Princeton, MA 01541 Page 1 Franz Fontaine Hornbeam 2"-2.5" $320.00 Carpinus betulus `Franz Fontaine` 2.5"-3" $460.00 3"-3.5" $620.00 American Hornbeam 2"-2.5" $300.00 Carpinus caroliniana 2.5"-3" $390.00 3"-3.5" $510.00 Northern Catalpa 2.5"-3" $360.00 Catalpa speciosa 3"-3.5" $470.00 3.5"-4" $690.00 4"-4.5" $900.00 Giant Dogwood 2"-2.5" $280.00 Cornus controversa `June Snow` 2.5"-3" $350.00 Tamarack 10'-12' $400.00 Larix laricina 12'-14' $530.00 (Spring dig only) 14'-16' $710.00 Crab Apple 1.5"-2" $210.00 Malus x `Donald Wyman` , `Schmidtcutleaf` TM, `Snowdrift` 2"-2.5" $260.00 American Hophornbeam -

Hornbeam Carpinus Betulus
34 W I N G E D S E E D S W I N G E D S E E D S 35 Sycamore Acer pseudoplatanus Hornbeam Carpinus betulus Leaves are large and five-lobed, with ornbeams are native trees found largely in south-eastern his introduced species grows in a wide dark green upper range of habitats and soil types. Sycamore sides.The seeds HEngland, with scattered trees in other parts of the country. T come in pairs, that They tolerate a wide range of soils, including sands, gravels and is an excellent coloniser and is often consid- are joined together ered a problem species as, in certain habitats, at an angle. heavy clay, but grow best on damp, fertile soils. Hornbeams including woodland, it can become the domi- produce excellent autumn colours, retaining their leaves throughout much of the winter. nant species. Large tree (5:10:25) Sycamore wood is light in colour, strong and One of the hardest and toughest woods in hard, and is used for kitchen table-tops, floor- Britain, the name hornbeam derives ing, veneers and toys. from the fact that the wood is as This species supports a limited number hard as horn. It was used for of insect species, which includes large cattle yokes, waterwheels and numbers of aphids. In consequence, butchers’ chopping blocks. The migrating warblers can often be found timber also makes excellent fire- feeding in sycamores in the autumn. The wood. tree also supports good lichen growth, Hornbeams are valu- particularly in the west of Britain. able to wildlife, pro- ducing nutlets Seed Guide: Collect the fruits from the which are eaten tree in autumn when they turn by hawfinches brown. -

SP532 Trees to Plant in Containers Or Wells
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Forestry, Trees, and Timber UT Extension Publications 3-1999 SP532 Trees to Plant In Containers or Wells The University of Tennessee Agricultural Extension Service Follow this and additional works at: https://trace.tennessee.edu/utk_agexfores Part of the Plant Sciences Commons Recommended Citation "SP532 Trees to Plant In Containers or Wells," The University of Tennessee Agricultural Extension Service, SP 532-15M-3/99 R12-4910-17-001-00, https://trace.tennessee.edu/utk_agexfores/54 The publications in this collection represent the historical publishing record of the UT Agricultural Experiment Station and do not necessarily reflect current scientific knowledge or ecommendations.r Current information about UT Ag Research can be found at the UT Ag Research website. This Trees for Tennessee Landscapes - Choosing the Right Tree is brought to you for free and open access by the UT Extension Publications at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Forestry, Trees, and Timber by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Agricultural Extension Service The University of Tennessee SP 532 Trees to Plant In Containers or Wells Donna C. Fare Wayne K. Clatterbuck Research Horticulturist Assistant Professor USDA-ARS Forestry, Wildlife US National Arboretum & Fisheries Donna C. Fare C. Donna Donna C. Fare C. Donna Pleasing example of a group of trees growing in containers. An in-ground tree planter with a magnolia tree. Landscaping in a small area is challenging, but popular. An important aspect of container or well planting is the Planting trees in small areas can limit root and shoot devel- soil or growing medium. -

Old-Growth Forests
Pacific Northwest Research Station NEW FINDINGS ABOUT OLD-GROWTH FORESTS I N S U M M A R Y ot all forests with old trees are scientifically defined for many centuries. Today’s old-growth forests developed as old growth. Among those that are, the variations along multiple pathways with many low-severity and some Nare so striking that multiple definitions of old-growth high-severity disturbances along the way. And, scientists forests are needed, even when the discussion is restricted to are learning, the journey matters—old-growth ecosystems Pacific coast old-growth forests from southwestern Oregon contribute to ecological diversity through every stage of to southwestern British Columbia. forest development. Heterogeneity in the pathways to old- growth forests accounts for many of the differences among Scientists understand the basic structural features of old- old-growth forests. growth forests and have learned much about habitat use of forests by spotted owls and other species. Less known, Complexity does not mean chaos or a lack of pattern. Sci- however, are the character and development of the live and entists from the Pacific Northwest (PNW) Research Station, dead trees and other plants. We are learning much about along with scientists and students from universities, see the structural complexity of these forests and how it leads to some common elements and themes in the many pathways. ecological complexity—which makes possible their famous The new findings suggest we may need to change our strat- biodiversity. For example, we are gaining new insights into egies for conserving and restoring old-growth ecosystems. canopy complexity in old-growth forests. -

Sass Forestecomgt 2018.Pdf
Forest Ecology and Management 419–420 (2018) 31–41 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Lasting legacies of historical clearcutting, wind, and salvage logging on old- T growth Tsuga canadensis-Pinus strobus forests ⁎ Emma M. Sassa, , Anthony W. D'Amatoa, David R. Fosterb a Rubenstein School of Environment and Natural Resources, University of Vermont, Burlington, VT 05405, USA b Harvard Forest, Harvard University, 324 N Main St, Petersham, MA 01366, USA ARTICLE INFO ABSTRACT Keywords: Disturbance events affect forest composition and structure across a range of spatial and temporal scales, and Coarse woody debris subsequent forest development may differ after natural, anthropogenic, or compound disturbances. Following Compound disturbance large, natural disturbances, salvage logging is a common and often controversial management practice in many Forest structure regions of the globe. Yet, while the short-term impacts of salvage logging have been studied in many systems, the Large, infrequent natural disturbance long-term effects remain unclear. We capitalized on over eighty years of data following an old-growth Tsuga Pine-hemlock forests canadensis-Pinus strobus forest in southwestern New Hampshire, USA after the 1938 hurricane, which severely Pit and mound structures damaged forests across much of New England. To our knowledge, this study provides the longest evaluation of salvage logging impacts, and it highlights developmental trajectories for Tsuga canadensis-Pinus strobus forests under a variety of disturbance histories. Specifically, we examined development from an old-growth condition in 1930 through 2016 across three different disturbance histories: (1) clearcut logging prior to the 1938 hurricane with some subsequent damage by the hurricane (“logged”), (2) severe damage from the 1938 hurricane (“hurricane”), and (3) severe damage from the hurricane followed by salvage logging (“salvaged”). -

The Impacts of Increasing Drought on Forest Dynamics, Structure, and Biodiversity in the United States
Global Change Biology (2016) 22, 2329–2352, doi: 10.1111/gcb.13160 SPECIAL FEATURE The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States JAMES S. CLARK1 , LOUIS IVERSON2 , CHRISTOPHER W. WOODALL3 ,CRAIGD.ALLEN4 , DAVID M. BELL5 , DON C. BRAGG6 , ANTHONY W. D’AMATO7 ,FRANKW.DAVIS8 , MICHELLE H. HERSH9 , INES IBANEZ10, STEPHEN T. JACKSON11, STEPHEN MATTHEWS12, NEIL PEDERSON13, MATTHEW PETERS14,MARKW.SCHWARTZ15, KRISTEN M. WARING16 andNIKLAUS E. ZIMMERMANN17 1Nicholas School of the Environment, Duke University, Durham, NC 27708, USA, 2Forest Service, Northern Research Station 359 Main Road, Delaware, OH 43015, USA, 3Forest Service 1992 Folwell Avenue,Saint Paul, MN 55108, USA, 4U.S. Geological Survey, Fort Collins Science Center Jemez Mountains Field Station, Los Alamos, NM 87544, USA, 5Forest Service, Pacific Northwest Research Station, Corvallis, OR 97331, USA, 6Forest Service, Southern Research Station, Monticello, AR 71656, USA, 7Rubenstein School of Environment and Natural Resources, University of Vermont, 04E Aiken Center, 81 Carrigan Dr., Burlington, VT 05405, USA, 8Bren School of Environmental Science and Management, University of California, Santa Barbara, CA 93106, USA, 9Department of Biology, Sarah Lawrence College, New York, NY 10708, USA, 10School of Natural Resources and Environment, University of Michigan, 2546 Dana Building, Ann Arbor, MI 48109, USA, 11U.S. Geological Survey, Southwest Climate Science Center and Department of Geosciences, University of Arizona, 1064 E. Lowell -

The Relationship Among Infection Intensity of Viscum Album with Some Ecological Parameters of Host Trees
Int. J. Environ. Res., 1(2): 143-149, Spring 2007 ISSN: 1735-6865 The Relationship among Infection Intensity of Viscum album with some Ecological Parameters of Host Trees Kartoolinejad, D.1*, Hosseini, S. M.1, Mirnia, S. K.2, Akbarinia, M.1 and Shayanmehr, F.1 1Natural Resources Faculty, Tarbiat Modares University, Tehran, Iran 2Agriculture Faculty, Tarbiat Modares University, Tehran, Iran Received 20 March 2006; Revised 12 Dec 2006; Accepted 10 Jan 2007 ABSTRACT: We investigated the relations among infection intensity of European mistletoe (Viscum album L.) with host tree features in Nour Forest Park, located in Caspian Forests in North of Iran. The number of 30 circular plots with an area of 0.1 ha were sampled in all places have an aggregation of infested trees. Parameters including DBH, height, distance to stand edge, distance to conspecific tree, bark diameter and the number of adult mistletoe per tree for all infected individuals were recorded. Results showed that the mistletoe abundance and infection intensity in Parrotia persica was more than the other host species and also, have positive significant relation with DBH, distance to conspecific and locating in the stand edge, but no significant relation observed about height of host trees. Results of this study suggest that individual differences among host trees (specially DBH) play an important role in explaining local abundance and distribution of mistletoe plants. Key words: Infection intensity, European mistletoe, Host trees, Caspian forests, Conspecific tree *Corresponding author: [email protected] INTRODUCTION Mistletoes are a polyphyletic and diverse real root, parasitize the stems of dicotyledonous group of flowering plants comprising over 1306 trees and shrubs by means of parenchyma organs species from a broad range of habitats across all named haustoria, developing from the radicle of continents except Antarctica. -

Ecological Insights from Long-Term Research Plots in Tropical and Temperate Forests
Ecological Insights from Long-term Research Plots in Tropical and Temperate Forests Organized Oral Session 40 was co-organized by Amy Wolf, Stuart Davies, and Richard Condit, and held on 6 August 2009 during the 94th ES A Annual Meeting in Albuquerque, New Mexico. An International Network of Forest Research Sites This session was devoted to findings from an international network of long-term forest dynamics plots, providing some of the first comparisons between the tropical and temperate study areas. Whereas most ecologists recognize the importance of long-term research (Callahan 1984, Likens 1989, Magnuson 1990, Hobbie et al. 2003, and others), large-scale projects like this one are rare. The global network of forest dynamics plots has expanded since its origin in the early 1980s, yet research at the plots has remained integrated due to a combination of individual scientists' efforts and institutional commitments from the Center for Tropical Forest Science (CTFS, <www.ctfs.si.edu> of the Smithsonian Tropical Research Institute and the Arnold Arboretum of Harvard University (Hubbell and Foster 1983, Condit 1995). Today, 26 large plots have been established in tropical or subtropical forests of Central and South America, Africa, Asia, and Oceania, along with 8 recently added temperate forest plots. A standard protocol (Condit 1998) is followed at all sites, including the marking, mapping, and measuring of all trees and shrubs with stem diameters >1 cm. Reports October 2009 519 Reports An underlying objective of the ESA session was to illustrate how an understanding of forest dynamics can contribute to long-term strategies of sustainable forest management in a changing global environment. -

Silvicultural Activities: Description and Terminology 1
WHITE PAPER F14-SO-WP-SILV-34 Silvicultural Activities: Description and Terminology 1 David C. Powell; Forest Silviculturist Supervisor’s Office; Pendleton, OR Initial Version: JUNE 1992 Most Recent Revision: FEBRUARY 2018 Introduction ........................................................................................................................ 2 Silvicultural systems ............................................................................................................ 2 Age-based silvicultural systems .................................................................................... 2 Even-aged management ............................................................................................... 3 Figure 1 – Three common stand structures ........................................................................... 4 Figure 2 – Clearcutting ............................................................................................................ 5 Figure 3 – Seed-tree and shelterwood cutting ....................................................................... 6 Figure 4 – Windthrow in spruce-fir forest in the northern Blue Mountains .......................... 8 Figure 5 – Examples of overstory removal and understory removal cutting ......................... 9 Uneven-aged management ........................................................................................ 10 Figure 6 – Examples of individual-tree selection cutting in ponderosa pine ....................... 12 Figure 7 – Examples of group selection -
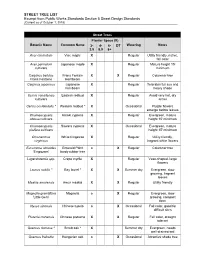
Approved Street Tree and Shrub List, from Public Works Standards
STREET TREE LIST Excerpt from Public Works Standards Section 5 Street Design Standards (Current as of October 1, 2019) Street Trees Planter Space (ft) Botanic Name Common Name 3- 4- 6- DT Watering Notes 3.9 5.9 8+ Acer circinatum Vine maple X Regular Utility friendly, native, fall color Acer palmatum Japanese maple X Regular Mature height 15' cultivars minimum Carpinus betulus Frans Fontain X X Regular Columnar tree Frans Fontaine Hornbeam Carpinus japonicus Japanese X Regular Tolerates full sun and hornbeam heavy shade Cercis canadensis Eastern redbud X Regular Avoid very hot, dry cultivars areas Cercis occidentalis * Western redbud * X Occasional Purple flowers emerge before leaves Chamaecyparis Hinoki cypress X Regular Evergreen, mature obtusa cultivars height 15' minimum Chamaecyparis Sawara cypress X Occasional Evergreen, mature pisifera cultivars height 15' minimum Chionanthus White fringetree X Regular Utility friendly, virginicus fragrant white flowers Eucommia ulmoides Emerald Point o X Regular Columnar tree 'Empozam' hardy rubber tree Lagerstroemia spp. Crepe myrtle X Regular Vase-shaped, large flowers Laurus nobilis * Bay laurel * X X Summer dry Evergreen, slow growing, fragrant leaves Maakia amurensis Amur maakia X X Regular Utility friendly Magnolia grandiflora Magnolia o X Regular Evergreen, slow 'Little Gem' growing, compact form Nyssa sinensis Chinese tupelo o X Occasional Fall color, good for difficult sites Pistacia chinensis Chinese pistache X X Regular Fall color, drought tolerant Quercus dumosa * Scrub oak * X Summer