Milia: a Review and Classification
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
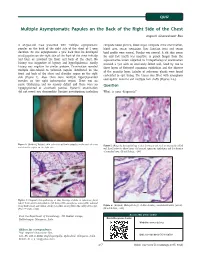
Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao
QUIZ Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao A 43-year-old male presented with multiple asymptomatic complete blood picture, blood sugar, complete urine examination, papules on the back of the right side of the chest of 1 year blood urea, serum creatinine, liver function tests and serum duration. He was asymptomatic a year back then he developed lipid profile were normal. Fundus was normal. A slit skin smear small papules on the right side of the front of the chest initially for acid fast bacilli was negative. A punch biopsy from the and later on involved the front and back of the chest. No representative lesion subjected to histopathological examination history was suggestive of leprosy and hyperlipidemias. Family revealed a cyst with an intricately folded wall, lined by two to history was negative for similar problem. Examination revealed three layers of flattened squamous epithelium and the absence multiple skin-colored to yellowish papules distributed on the of the granular layer. Lobules of sebaceous glands were found front and back of the chest and shoulder region on the right embedded in cyst lining. The lumen was filled with amorphous side [Figure 1]. Also, there were multiple hyperpigmented eosinophilic material and multiple hair shafts [Figures 2-4]. macules on the right infrascapular region. There was no nerve thickening and no sensory deficit and there were no Question hypopigmented or anesthetic patches. Systemic examination did not reveal any abnormality. Routine investigations including What is your diagnosis? (Original) Multiple skin-colored to yellowish papules on the back of chest Figure 1: Figure 2: (Original) Histopathology of skin showing a cyst with an intricately folded and shoulder region on the right side wall lined by two to three layers of flattened squamous epithelium and the absence of granular layer. -

Neonatal Dermatology Review
NEONATAL Advanced Desert DERMATOLOGY Dermatology Jennifer Peterson Kevin Svancara Jonathan Bellew DISCLOSURES No relevant financial relationships to disclose Off-label use of acitretin in ichthyoses will be discussed PHYSIOLOGIC Vernix caseosa . Creamy biofilm . Present at birth . Opsonizing, antibacterial, antifungal, antiparasitic activity Cutis marmorata . Reticular, blanchable vascular mottling on extremities > trunk/face . Response to cold . Disappears on re-warming . Associations (if persistent) . Down syndrome . Trisomy 18 . Cornelia de Lange syndrome PHYSIOLOGIC Milia . Hard palate – Bohn’s nodules . Oral mucosa – Epstein pearls . Associations . Bazex-Dupre-Christol syndrome (XLD) . BCCs, follicular atrophoderma, hypohidrosis, hypotrichosis . Rombo syndrome . BCCs, vermiculate atrophoderma, trichoepitheliomas . Oro-facial-digital syndrome (type 1, XLD) . Basal cell nevus (Gorlin) syndrome . Brooke-Spiegler syndrome . Pachyonychia congenita type II (Jackson-Lawler) . Atrichia with papular lesions . Down syndrome . Secondary . Porphyria cutanea tarda . Epidermolysis bullosa TRANSIENT, NON-INFECTIOUS Transient neonatal pustular melanosis . Birth . Pustules hyperpigmented macules with collarette of scale . Resolve within 4 weeks . Neutrophils Erythema toxicum neonatorum . Full term . 24-48 hours . Erythematous macules, papules, pustules, wheals . Eosinophils Neonatal acne (neonatal cephalic pustulosis) . First 30 days . Malassezia globosa & sympoidalis overgrowth TRANSIENT, NON-INFECTIOUS Miliaria . First weeks . Eccrine -

Bilateral Lower Extremity Hyperkeratotic Plaques: a Case Report of Ichthyosis Vulgaris
Faculty & Staff Scholarship 2015 Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Zachary Zinn Omid Jalali Follow this and additional works at: https://researchrepository.wvu.edu/faculty_publications Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Abstract: Here, we report a case of a middle-aged woman presenting with severe, long-standing, Zachary Zinn hyperkeratotic plaques of the lower extremities unrelieved by over-the-counter medications. Omid Jalali Initial history and clinical findings were suggestive of an inherited ichthyosis. Ichthyoses are genetic disorders characterized by dry scaly skin and altered skin-barrier function. A diagnosis Department of Dermatology, West Virginia University, of ichthyosis vulgaris was confirmed by histopathology. Etiology, prevalence, and treatment Morgantown, WV, USA options are discussed. Keywords: filaggrin gene, FLG, profilaggrin, keratohyalin granules, hyperkeratosis Introduction For personal use only. Inherited ichthyoses are a diverse group of genetic disorders characterized by dry, scaly skin; hyperkeratosis; and altered skin-barrier function. While these disorders of cutaneous keratinization are multifaceted and varying in etiology, disruption in the stratum corneum with generalized scaling is common to all.1–4 Although not entirely known -

Revertant Mosaicism in a Human Skin Fragility Disorder Results from Slipped Mispairing and Mitotic Recombination
Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi, … , Leena Bruckner-Tuderman, Cristina Has J Clin Invest. 2012;122(5):1742-1746. https://doi.org/10.1172/JCI61976. Brief Report Dermatology Spontaneous gene repair, also called revertant mosaicism, has been documented in several genetic disorders involving organs that undergo self-regeneration, including the skin. Genetic reversion may occur through different mechanisms, and in a single individual, the mutation can be repaired in various ways. Here we describe a disseminated pattern of revertant mosaicism observed in 6 patients with Kindler syndrome (KS), a genodermatosis caused by loss of kindlin-1 (encoded by FERMT1) and clinically characterized by patchy skin pigmentation and atrophy. All patients presented duplication mutations (c.456dupA and c.676dupC) in FERMT1, and slipped mispairing in direct nucleotide repeats was identified as the reversion mechanism in all investigated revertant skin spots. The sequence around the mutations demonstrated high propensity to mutations, favoring both microinsertions and microdeletions. Additionally, in some revertant patches, mitotic recombination generated areas with homozygous normal keratinocytes. Restoration of kindlin-1 expression led to clinically and structurally normal skin. Since loss of kindlin-1 severely impairs keratinocyte proliferation, we predict that revertant cells have a selective advantage that allows their clonal expansion and, consequently, the improvement of the skin condition. Find the latest version: https://jci.me/61976/pdf Brief report Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi,1 Yinghong He,1 Anna M.G. Pasmooij,2 Meltem Onder,3 Rudolf Happle,1 Marcel F. -

Skin Brief Articles
SKIN BRIEF ARTICLES Nab-paclitaxel/gemcitabine Induced Acquired Ichthyosis Adriana Lopez BAa, Joel Shugar MDb, and Mark Lebwohl MDc aColumbia University Vagelos College of Physicians and Surgeons, New York, NY bIcahn School of Medicine at Mount Sinai, Department of Otolaryngology, New York, NY cIcahn School of Medicine at Mount Sinai, Department of Dermatology, New York, NY ABSTRACT The ichthyoses are a diverse group of cutaneous disorders characterized by abnormalities in cornification. The majority of ichthyoses are inherited with childhood presentation and new onset ichthyosis in adulthood warrants further medical evaluation. Though most well recognized for its association with Hodgkin’s disease, acquired ichthyosis (AI) has been linked to a number of inflammatory, autoimmune, and endocrine processes. However, drug- induced AI is exceedingly rare and remains a poorly understood entity. Here we report a case of a male patient who developed AI while receiving nab-paclitaxel plus gemcitabine for treatment of pancreatic adenocarcinoma. months prior, the patient was first seen for INTRODUCTION recurrent, self-healing, pruritic erythematous Acquired ichythyosis (AI) is an uncommon papules. Punch biopsy was performed which non-inherited cutaneous disorder of showed an atypical cellular infiltrate of abnormal keratinization that is most scattered large CD30+ cells with clonal T-cell frequently associated with underlying receptor-β gene rearrangement. Though the malignancy. Drug induced AI is uncommon clinicopathologic diagnosis was most and has been rarely linked to consistent with lymphomatoid papulosis chemotherapeutic agents. Herein, we report (LyP), imaging was pursued to exclude the case of a man with pancreatic extracutaneous lymphoproliferative disease. adenocarcinoma who developed an CT scan incidentally detected a mass in the ichthyosiform eruption upon starting body of the pancreas and biopsy was chemotherapy with nab-paclitaxel plus concordant with pancreatic adenocarcinoma. -

Lymphatic Complaints in the Dermatology Clinic: an Osteopathic
Volume 35 JAOCDJournal Of The American Osteopathic College Of Dermatology Lymphatic Complaints in the Dermatology Clinic: An Osteopathic Approach to Management A five-minute treatment module makes lymphatic OMT a practical option in busy practices. Also in this issue: A Case of Acquired Port-Wine Stain (Fegeler Syndrome) Non-Pharmacologic Interventions in the Prevention of Pediatric Atopic Dermatitis: What the Evidence Says Inflammatory Linear Verrucous Epidermal Nevus Worsening in Pregnancy last modified on June 9, 2016 10:54 AM JOURNAL OF THE AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY Page 1 JOURNAL OF THE AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY 2015-2016 AOCD OFFICERS PRESIDENT Alpesh Desai, DO, FAOCD PRESIDENT-ELECT Karthik Krishnamurthy, DO, FAOCD FIRST VICE-PRESIDENT Daniel Ladd, DO, FAOCD SECOND VICE-PRESIDENT John P. Minni, DO, FAOCD Editor-in-Chief THIRD VICE-PRESIDENT Reagan Anderson, DO, FAOCD Karthik Krishnamurthy, DO SECRETARY-TREASURER Steven Grekin, DO, FAOCD Assistant Editor TRUSTEES Julia Layton, MFA Danica Alexander, DO, FAOCD (2015-2018) Michael Whitworth, DO, FAOCD (2013-2016) Tracy Favreau, DO, FAOCD (2013-2016) David Cleaver, DO, FAOCD (2014-2017) Amy Spizuoco, DO, FAOCD (2014-2017) Peter Saitta, DO, FAOCD (2015-2018) Immediate Past-President Rick Lin, DO, FAOCD EEC Representatives James Bernard, DO, FAOCD Michael Scott, DO, FAOCD Finance Committee Representative Donald Tillman, DO, FAOCD AOBD Representative Michael J. Scott, DO, FAOCD Executive Director Marsha A. Wise, BS AOCD • 2902 N. Baltimore St. • Kirksville, MO 63501 800-449-2623 • FAX: 660-627-2623 • www.aocd.org COPYRIGHT AND PERMISSION: Written permission must be obtained from the Journal of the American Osteopathic College of Dermatology for copying or reprinting text of more than half a page, tables or figures. -

Basal Cell Carcinoma: Topical Therapy Versus Surgical Treatment
Journal of the Saudi Society of Dermatology & Dermatologic Surgery (2012) 16,41–51 King Saud University Journal of the Saudi Society of Dermatology & Dermatologic Surgery www.ksu.edu.sa www.jssdds.org www.sciencedirect.com REVIEW ARTICLE Basal cell carcinoma: Topical therapy versus surgical treatment Khalifa E. Sharquie a,*, Adil A. Noaimi b,1 a Scientific Council of Dermatology and Venereology, Iraqi Board for Medical Specializations, Iraq b Department of Dermatology and Venereology, College of Medicine, University of Baghdad, Iraq Received 1 November 2011; revised 7 June 2012; accepted 7 June 2012 Available online 6 July 2012 KEYWORDS Abstract Basal cell carcinoma (BCC) is a non-melanoma skin cancer and is one of the commonest Basal cell carcinoma; malignancies of the skin encountered in daily clinical practice. It derives from non-keratinizing cells Topical therapy of BCC by that originate in the basal layer of the epidermis. It accounts for approximately 75% of all skin can- podophylline; cer in the world, and 53.2% in Iraqi patients. Generally, most BCC cases are sporadic, but it may Surgical treatment also appear in genetic disorders such as Gorlin’s syndrome, Rombo’s syndrome, and xeroderma pigmentosa (XP). If left untreated, BCC will continue to invade locally and may result in substantial tissue damage that compromises function and cosmetic look. Metastasis is an extremely rare event. The tumor characteristically develops on sun-exposed skin of lighter-skinned individuals. There are two main modalities of therapy including topical like: Imiquimod, 5-fluorouracil (5- FU), photodynamic therapy (PDT), radiation therapy (RT), topical & intralesional zinc sulfate and topical 25% podophylline. -

Northwestern University, October 2003
CHICAGO DERMATOLOGICAL SOCIETY CASE # 1 Presented by James Swan, M.D., Mary Martini, M.D. and Keren Horn, M.D. Department of Dermatology, Feinberg School of Medicine, Northwestern University HISTORY OF PRESENT ILLNESS This 45 year-old Caucasian male presented for a problem-focused exam of a right upper extremity lesion. However, complete physical exam revealed peri-orbital slate-gray hyperpigmentation as well as generalized bronze hyperpigmentation in sun-exposed areas, prompting questions regarding these abnormalities. Other abnormalities in pigment included approximately 10 café au lait macules, nearly all greater than 1.5cm in size. Axillary freckling was also noted. Review of systems was positive for migraine headaches, arthritis, decreased libido and fatigue (the patient sleeps greater than 12 hours each night without feeling refreshed). PAST MEDICAL HISTORY Right orbital tumor status post excision 2001 Depression MEDICATIONS Depakote Remeron ALLERGIES Amoxicillin (angioedema) FAMILY HISTORY Brother with the same pigmentation anomalies. Both his father and his sister died of liver cancer; his sister passed away at 40 years of age. SOCIAL HISTORY The patient does not drink alcohol and has smoked one pack of cigarettes per day for the past 30 years. He is on disability due to “depression” and exhaustion which make it unable for him to sustain an eight hour work day. PHYSICAL EXAM There is peri-orbital slate-gray hyperpigmentation as well as generalized bronze hyperpigmentation in sun-exposed areas. In addition, the patient has approximately 10 café au lait macules, nearly all greater than 1.5cm in size. Axillary freckling is also noted, and there are protuberances of the left elbow and right knee. -

Cytoplasmic Plaque Formation in Hemidesmosome Development Is Dependent on Soxf Transcription Factor Function
Cytoplasmic Plaque Formation in Hemidesmosome Development Is Dependent on SoxF Transcription Factor Function Shelly Oommen1, Mathias Francois2, Maiko Kawasaki1, Melanie Murrell2, Katsushige Kawasaki1, Thantrira Porntaveetus1, Sarah Ghafoor1, Neville J. Young2, Yoshimasa Okamatsu3, John McGrath4, Peter Koopman2, Paul T. Sharpe1, Atsushi Ohazama1,3* 1 Craniofacial Development and Stem Cell Biology, and Biomedical Research Centre, Dental Institute, King’s College London, London, United Kingdom, 2 Institute for Molecular Bioscience, The University of Queensland, Brisbane, Australia, 3 Department of Periodontology, Showa University Dental School, Tokyo, Japan, 4 Genetic Skin Disease Group, St John’s Institute of Dermatology, Division of Skin Sciences, King’s College London, London, United Kingdom Abstract Hemidesmosomes are composed of intricate networks of proteins, that are an essential attachment apparatus for the integrity of epithelial tissue. Disruption leads to blistering diseases such as epidermolysis bullosa. Members of the Sox gene family show dynamic and diverse expression patterns during development and mutation analyses in humans and mice provide evidence that they play a remarkable variety of roles in development and human disease. Previous studies have established that the mouse mutant ragged-opossum (Raop) expresses a dominant-negative form of the SOX18 transcription factor that interferes with the function of wild type SOX18 and of the related SOXF-subgroup proteins SOX7 and 217. Here we show that skin and oral mucosa in homozygous Raop mice display extensive detachment of epithelium from the underlying mesenchymal tissue, caused by tearing of epithelial cells just above the plasma membrane due to hemidesmosome disruption. In addition, several hemidesmosome proteins expression were found to be dysregulated in the Raop mice. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Dumitras, Cu, MC; Popa, A.; Petca, A.; Miulescu, R.-G. Cutaneous Mastocytosis in Childhood—Update from the Literature
Journal of Clinical Medicine Review Cutaneous Mastocytosis in Childhood—Update from the Literature 1,2 1,3 1,4 1,5, Florica Sandru ,Răzvan-Cosmin Petca , Monica Costescu , Mihai Cristian Dumitras, cu *, Adelina Popa 2, Aida Petca 1,6,* and Raluca-Gabriela Miulescu 1,7 1 “Carol Davila” University of Medicine and Pharmacy, 030167 Bucharest, Romania; fl[email protected] (F.S.); [email protected] (R.-C.P.); [email protected] (M.C.); [email protected] (R.-G.M.) 2 Department of Dermatology, Elias University Emergency Hospital, 0611461 Bucharest, Romania; [email protected] 3 Department of Urology, “Prof. Dr. Theodor Burghele” Clinical Hospital, 061344 Bucharest, Romania 4 Department of Dermatology, “Dr. Victor Babes” Clinical Hospital of Infectious and Tropical Diseases, 030303 Bucharest, Romania 5 Department of Obstetrics & Gynecology, University Emergency Hospital, 050098 Bucharest, Romania 6 Department of Obstetrics & Gynecology, Elias University Emergency Hospital, 0611461 Bucharest, Romania 7 Department of Dermatology, Vălenii de Munte Hospital, 106400 Prahova, Romania * Correspondence: [email protected] (M.C.D.); [email protected] (A.P.); Tel.: +40-722-223223 (M.C.D.); +40-745-787448 (A.P.) Abstract: Mastocytosis (M) represents a systemic pathology characterized by increased accumulation and clonal proliferation of mast cells in the skin and/or different organs. Broadly, M is classified into two categories: Cutaneous mastocytosis (CM) and systemic mastocytosis (SM). In children, CM is Citation: Sandru, F.; Petca, R.-C.; the most frequent form. Unfortunately, pathogenesis is still unclear. It is thought that genetic factors Costescu, M.; Dumitras, cu, M.C.; are involved, but further studies are necessary. As for features of CM, the lesions differ in clinical Popa, A.; Petca, A.; Miulescu, R.-G. -

Pili Torti: a Feature of Numerous Congenital and Acquired Conditions
Journal of Clinical Medicine Review Pili Torti: A Feature of Numerous Congenital and Acquired Conditions Aleksandra Hoffmann 1 , Anna Wa´skiel-Burnat 1,*, Jakub Z˙ ółkiewicz 1 , Leszek Blicharz 1, Adriana Rakowska 1, Mohamad Goldust 2 , Małgorzata Olszewska 1 and Lidia Rudnicka 1 1 Department of Dermatology, Medical University of Warsaw, Koszykowa 82A, 02-008 Warsaw, Poland; [email protected] (A.H.); [email protected] (J.Z.);˙ [email protected] (L.B.); [email protected] (A.R.); [email protected] (M.O.); [email protected] (L.R.) 2 Department of Dermatology, University Medical Center of the Johannes Gutenberg University, 55122 Mainz, Germany; [email protected] * Correspondence: [email protected]; Tel.: +48-22-5021-324; Fax: +48-22-824-2200 Abstract: Pili torti is a rare condition characterized by the presence of the hair shaft, which is flattened at irregular intervals and twisted 180◦ along its long axis. It is a form of hair shaft disorder with increased fragility. The condition is classified into inherited and acquired. Inherited forms may be either isolated or associated with numerous genetic diseases or syndromes (e.g., Menkes disease, Björnstad syndrome, Netherton syndrome, and Bazex-Dupré-Christol syndrome). Moreover, pili torti may be a feature of various ectodermal dysplasias (such as Rapp-Hodgkin syndrome and Ankyloblepharon-ectodermal defects-cleft lip/palate syndrome). Acquired pili torti was described in numerous forms of alopecia (e.g., lichen planopilaris, discoid lupus erythematosus, dissecting Citation: Hoffmann, A.; cellulitis, folliculitis decalvans, alopecia areata) as well as neoplastic and systemic diseases (such Wa´skiel-Burnat,A.; Zółkiewicz,˙ J.; as cutaneous T-cell lymphoma, scalp metastasis of breast cancer, anorexia nervosa, malnutrition, Blicharz, L.; Rakowska, A.; Goldust, M.; Olszewska, M.; Rudnicka, L.