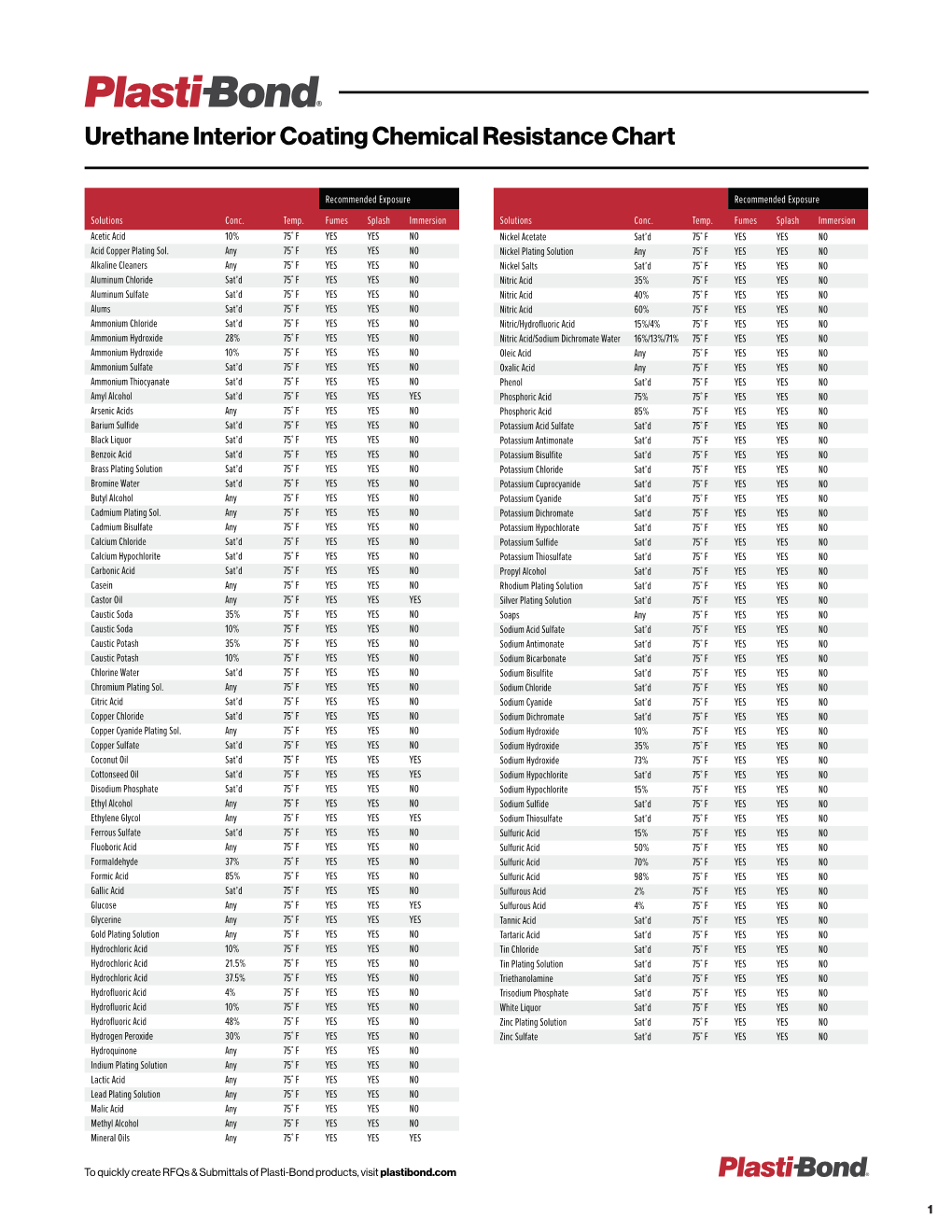
Urethane Interior Coating Chemical Resistance Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) (11) 4,078,917 Swanson 45) Mar
United States Patent (19) (11) 4,078,917 Swanson 45) Mar. 14, 1978 54 EXTRACTION OF ANTIMONY TRIOXIDE from antimony sulfide ore concentrate by solubility FROMANTIMONY SULFDE ORE differential of the trioxide in lower alkanol solutions of 76 Inventor: Rollan Swanson, The Baker House, sodium or potassium hydroxide and wherein the total 220 California Ave., Santa Monica, amount of water contained in the concentrate, the alka Calif. 95405 nol and the hydroxide is not more than 26.52 volume percent of the antimony sulfide content; which process (21) Appl. No.: 652,093 includes treating the ore in the absence of substantial (22) Filed: Jan. 26, 1976 amounts of air with an alkanol solution containing an excess of sodium or potassium hydroxide, basis Sb2S3 51) Int. Cl’.............................................. C22B 30/02 content in the ore; separating also, in the absence of 52 U.S. C. .................................... 7.5/101 R; 7.5/108; substantial amounts of air, insoluble concentrate mate 75/121; 42.3/87; 423/617 rial from a filtrate composed of water, alkanol, hydrox 58) Field of Search ..................... 75/101 R, 121, 108; ide and sulfide of potassium or sodium, antimony triox 423/87, 617 ide trihydrate, sodium or potassium dihydro pyroan (56) References Cited timonite; repeatedly extracting the insoluble material U.S. PATENT DOCUMENTS with the filtrate also in the absence of substantial 796,849 8/1905 MacArthur ........................ 75/121 X amounts of air; allowing the filtrate to settle so as to 975,148 ill/1910 Masson .................................. 75/121 form a precipitate of antimony trioxide and sodium or 1,548,854 8/1925 Schleicher . -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Pd and Cu-MEDIATED DOMINO REACTIONS for THE
254 Pd AND Cu-MEDIATED DOMINO REACTIONS FOR THE SYNTHESIS OF SULFUR HETEROCYCLES DOI: http://dx.medra.org/10.17374/targets.2018.21.254 Morgan Donnard,* Thomas Castanheiro and Mihaela Gulea* CNRS, University of Strasbourg, LIT UMR 7200, 67000 Strasbourg, France (e-mail: [email protected]; [email protected]) Abstract. Pd and Cu-mediated domino reactions are powerful tools for the synthesis of heterocycles. In this chapter we have compiled the most relevant examples of such transformations for the synthesis of sulfur- containing heterocycles. These sequences have been organized by type of heterocycle formed and the nature of the substrates (with or without a sulfur atom). Contents 1. Introduction 2. Synthesis of heterocycles incorporating only sulfur heteroatom 2.1. From sulfur-containing starting material 2.2. From sulfur-free starting material 3. Synthesis of heterocycles incorporating one sulfur and at least another heteroatom 3.1. N,S-Heterocycles 3.1.1. From sulfur-containing starting material 3.1.2 From sulfur-free starting material 3.2 O,S-Heterocycles 3.2.1. From sulfur-containing starting material 3.2.2. From sulfur-free starting material 4. Conclusion Acknowledgements References 1. Introduction The impact of organosulfur compounds in pharmaceutical industry is significant, several sulfur- containing drugs being among the most prescribed and sold at present in the world.1 Some of them contain in their structure a sulfur heterocycle (Figure 1). Considering the continual need for the development of medicinal therapies, new S-heterocyclic compounds could lead to forthcoming pharmaceuticals. Figure 1. Structures of drugs containing a sulfur-heterocycle. 255 As a consequence, the search for new syntheses to provide original compounds of these types represents a topical subject. -

Potash Sulfurated 1
MSDS Number: P5359 * * * * * Effective Date: 03/28/11 * * * * * Supersedes: 11/21/08 POTASH SULFURATED 1. Product Identification Synonyms: Liver of sulfur; sulfurated potassa; hepar sulfuris CAS No.: 39365-88-3 (Potash) Molecular Weight: Not applicable to mixtures. Chemical Formula: Not applicable. Product Codes: J.T. Baker: 2909 Macron: 7155 2. Composition/Information on Ingredients Ingredient CAS No Percent Hazardous --------------------------------------- ------------ ------------ --------- Potassium Trisulfide 37488-75-8 25% Yes Potassium Thiosulfate Hydrated 10294-66-3 75% Yes 3. Hazards Identification Emergency Overview -------------------------- WARNING! FLAMMABLE SOLID. HARMFUL IF SWALLOWED OR INHALED. CAUSES IRRITATION TO SKIN, EYES AND RESPIRATORY TRACT. DUST MAY FORM FLAMMABLE OR EXPLOSIVE MIXTURE WITH AIR. SAF-T-DATA(tm) Ratings (Provided here for your convenience) ----------------------------------------------------------------------------------------------------------- Health Rating: 2 - Moderate Flammability Rating: 3 - Severe (Flammable) Reactivity Rating: 2 - Moderate Contact Rating: 1 - Slight Lab Protective Equip: GOGGLES; LAB COAT; VENT HOOD; PROPER GLOVES; CLASS D EXTINGUISHER Storage Color Code: Red (Flammable) ----------------------------------------------------------------------------------------------------------- Potential Health Effects ---------------------------------- Inhalation: Inhalation of dust can irritate the respiratory tract. Production of hydrogen sulfide from reaction with acids or high temperature -
(12) United States Patent (10) Patent No.: US 7,862,708 B2 Siskin Et Al
US007862708B2 (12) United States Patent (10) Patent No.: US 7,862,708 B2 Siskin et al. (45) Date of Patent: Jan. 4, 2011 (54) PROCESS FOR THE DESULFURIZATION OF 2005/0133405 A1 6/2005 Wellington et al. HEAVY OLS AND BITUMENS 2005/0145536 A1 7/2005 Wellington et al. 2005/O155906 A1 7/2005 Wellington et al. (75) Inventors: Michael Siskin, Westfield, NJ (US); 2007/0102323 A1 5/2007 Lee et al. Rustom M. Billimoria, Hellertown, PA FOREIGN PATENT DOCUMENTS (US); David W. Savage, Zionsville, IN (US); Roby Bearden, Jr., Baton Rouge, BE 888.421 10, 1981 LA (US) WO WO99/15605 4f1999 WO WO 2005/061665 A2 7/2005 Assignee: ExxonMobil Research and WO WO 2005/061670 A2 7/2005 (73) WO WO 2005/061671 A2 7/2005 Engineering Company, Annandale, NJ WO WO 2005/063936 A2 7/2005 (US) WO WO 2005/066304 A2 7/2005 WO WO 2005/066305 A2 7/2005 (*) Notice: Subject to any disclaimer, the term of this WO WO 2005/066309 A2 7/2005 patent is extended or adjusted under 35 U.S.C. 154(b) by 113 days. OTHER PUBLICATIONS Claudio Bianchini, Andrea Meli; “Hydrogenation, hydrogenolysis, (21) Appl. No.: 12/287,744 and hydrodesulfurization of thiophenes.” Multiphase Homogeneous Catalysis (2005), vol. 1, 196-202. Abstract. (22) Filed: Oct. 14, 2008 Atsushi Kishita, Satoru Takahashi, Hirotaka Kamimura, Masami Miki, Takehiko Morita, Heiji Enomoto; “Upgrading of Bitumen by (65) Prior Publication Data Hydrothermal Visbreaking in Supercritical Water with Alkali.” Regu US 2009/O152168A1 Jun. 18, 2009 lar Paper, Graduate School of Environmental Studies, Tohoku Uni versity, Sendai, Japan. -

D I C I O N Á R I O De Q U Í M I Ca
7 2 8 2 53 2 5 6 6 18 18 d I 7 c i o N á r i O Iodo Nitrogênio Oxigênio 126.90447 14.0067 126.90447 de 92 2 11 2 8 8 18 8 32 2 21 9 Q U 2 í m i Ca Urânio Cálcio 238.02891 40.078 Inglês - Português Fernando José Luna DICIONÁRIO DE QUÍMICA INGLÊS - PORTUGUÊS 0 8 Fernando José Luna 0 8 Seropédica 2019 Reitor Ricardo Luiz Louro Berbara Vice-Reitor Luiz Carlos de Oliveira Lima Pró-Reitor de Pesquisa e Pós-Graduação Alexandre Fortes Pró-Reitora Adjunta de Pesquisa e Pós-Graduação Lucia Helena Cunha dos Anjos Conselho Consultivo: Adriana T. M. Lessa Lúcia Valadares Sartório Ana Maria Marques dos Santos Luiz Alberto de Lima Leandro Ana Paula Perrota Franco Manlio Silvestre Fernandes Biancca Scarpeline de Castro Márcio Rufino Silva Carmen Andriolli Maria Gracinda Carvalho Teixeira Christian Dutilleux Marta Cioccari Cláudia Mazza Rebeca Gontijo Teixeira Clézio dos Santos Simone Batista Danilo Bilate Tania Mikaela Garcia Roberto Débora Lerrer Vladimyr Lombardo Jorge Janaína Machado Simões Yllan de Mattos Oliveira Lígia Fátima Lima Calixto EDUR Editora da Universidade Federal Rural do Rio de Janeiro Br 465, Km. 7, Seropédica – RJ - CEP: 23.897-000 Telefone: (21) 2681-4711 Site: www.editora.ufrrj.br E-mail: [email protected] Relação das abreviações usadas neste dicionário: abr., abrev. abreviação adj. adjetivo LALQ alquimia amer. inglês americano BIO biologia BIOTEC biotecnologia CATAL catálise cf. confira CG cromatografia a gás CROMAT cromatografia DH Dicionário Houaiss ENG.QUÍM engenharia química FÍS física FOTQ fotoquímica G.QUÍM guerra química GEOQUÍM geoquímica ing. -

Process for Preparing Arylene Sulfide Polymers
Europaisches Patentamt European Patent Office © Publication number: 0 486 014 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91119439.7 int. a* C08G 75/02 @ Date of filing: 14.11.91 © Priority: 14.11.90 US 612658 @ Inventor: Ash, Carlton Edwin 4600 Stonehenge Drive @ Date of publication of application: Bartlesville, OK 74006(US) 20.05.92 Bulletin 92/21 © Representative: Dost, Wolfgang, © Designated Contracting States: Dr.rer.nat.,Dipl.-Chem. et al AT BE CH DE DK ES FR GB GR IT LI LU NL SE Patent- & Rechtsanwalte Bardehle . Pagenberg . Dost . Altenburg . Frohwitter . © Applicant: PHILLIPS PETROLEUM COMPANY Geissler & Partner Galileiplatz 1 Postfach 86 5th and Keeler 06 20 Bartlesville Oklahoma 74004(US) W-8000 Munchen 86(DE) © Process for preparing arylene sulfide polymers. © An arylene sulfide polymer and a process for preparing same by contacting a sulfur source, a cyclic organic amide and a dihaloaromatic compound to produce a polymerization mixture, polymerizing the polymerization mixture and recovering the arylene sulfide polymer wherein the process is conducted in the presence of a compound having the formula wherein X is selected from the group consisting of bromine and iodine, Y is a non-activating or deactivating group and is selected from the group consisting of -R', -OR', -Ar', -OAr', -CT, /A < -NR'2 CO 00 ^ -NR"Cit V Rank Xerox (UK) Business Services (3.08/2. 19/2.0) EP 0 486 014 A1 -SiR's and -R"SiR'3 , R' and R" are hydrogen or an alkyl group having 1 to about 15 carbon atoms, and Ar' is selected from the group consisting of 2 EP 0 486 014 A1 Background of the Invention This invention relates to the production of arylene sulfide polymers. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Spent Metal Catalyst Other means of identification SDS number 901 - GHS Synonyms Spent metal catalyst. See section 16 for complete information. Recommended use This product is intended for use as a refinery feedstock, fuel or for use in engineered processes. Use in other applications may result in higher exposures and require additional controls, such as local exhaust ventilation and personal protective equipment. Recommended restrictions None known. Manufacturer/Importer/Supplier/Distributor information Manufacturer/Supplier Valero Marketing & Supply Company and Affiliates One Valero Way San Antonio, TX 78269-6000 General Assistance 210-345-4593 E-Mail [email protected] Contact Person Industrial Hygienist Emergency Telephone 24 Hour Emergency 866-565-5220 1-800-424-9300 (CHEMTREC USA) 2. Hazard(s) identification Physical hazards Self-heating substances and mixtures Category 1 Health hazards Acute toxicity, oral Category 3 Acute toxicity, inhalation Category 3 Skin corrosion/irritation Category 1A Serious eye damage/eye irritation Category 1 Sensitization, respiratory Category 1 Sensitization, skin Category 1 Germ cell mutagenicity Category 2 Carcinogenicity Category 1A Reproductive toxicity Category 2 Specific target organ toxicity, single exposure Category 3 respiratory tract irritation Specific target organ toxicity, repeated Category 1 (lung, respiratory system) exposure Environmental hazards Hazardous to the aquatic environment, acute Category 1 hazard Hazardous to the aquatic environment, Category 1 long-term hazard OSHA defined hazards Combustible dust Label elements Signal word Danger Hazard statement Toxic if swallowed. Toxic if inhaled. Causes severe skin burns and eye damage. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause an allergic skin reaction. -

HDPE Chemical Resistance Guide
HDPE Chemical Resistance Guide 70º F 140º F 70º F 140º F Reagent (21º C) (60º C) Reagent (21º C) (60º C) A B Acetaldehyde S O Barium carbonate saturated S S Acetic acid (1-10%) S S Barium carbonate saturated S S Acetic acid (10-60%) S O Barium hydroxide S S Acetic acid (80-100%) S O Barium sulfate saturated S S Acetic anhydride S S Barium sulfite saturated S S Acetone S S Beer S S Acids (aromatic) S S Benzaldehyde S O Acrylic emulsions S S Benzene O U Adipic acid S S Benzene sulfonic acid S S Aluminum chloride concentrated S S Benzoic acid crystals S S Aluminum chloride dilute S S Benzoic acid saturated S S Aluminum fluoride concentrated S S Bismuth carbonate saturated S S Aluminum sulfate concentrated S S Black liquor S S Alums (all types) concentrated S S Bleach lye (10%) S S Amino acetic acid S S Borax cold saturated S S Ammonia (100% dry gas) S S Boric acid concentrated S S Ammonium acetate S S Boric acid dilute S S Ammonium bromide S S Brine S S Ammonium carbonate S S Bromic acid (10%) S S Ammonium chloride saturated S S Bromine liquid (100%) O U Ammonium fluoride (20%) S S Bromochloromethane U U Ammonium hydroxide S S Butadiene U U Ammonium metaphosphate (sat.) S S Butanediol (10%) S S Ammonium nitrate saturated S S Butanediol (60%) S S Ammonium persulfate saturated S S Butanediol (100%) S S Ammonium phosphate S S Butter S S Ammonium sulfate saturated S S Butyl acetate (100%) O U Ammonium sulfide saturated S S Butyl alcohol (100%) S S Ammonium thiocyanate saturated S S Butylene glycol S S Amyl acetate (100%) O U Butyric acid (100%) -
The Influence of Chemicals on the Growth of Bacteria
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1932 The Influence of Chemicals on the Growth of Bacteria Alice J. Burke Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Bacteriology Commons Recommended Citation Burke, Alice J., "The Influence of Chemicals on the Growth of Bacteria" (1932). Master's Theses. 13. https://ecommons.luc.edu/luc_theses/13 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1932 Alice J. Burke LOYOLA UNIVERSITY THE INFLUENCE OF CHEMICALS ON THE GROWTH OF BACTERIA A THESIS SUBMITTED TO THE FACULTY OF LOYOLA UNIVERSITY GRADUATE SCHOOL m CANDIDACY FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF BACTERIOLOGY BY ALICE J. BURKE CHICAGO, ILLINOIS 1932. 1. INTRODUCTION. In presenting the paper as a resume of an investigation with regards to the influence of chemicals on the selective growth of bacteria I wish to acknowledge my great indebtedness to Dr. Emil Weiss, Professor of Bacteriol~gy at Loyola Univer sity School of Medicine, who suggested the subject. I am also indebted to Dr. Weiss for his conatant interest and kind super vision of this work. This project, as a whole, is a continua tion of an earlier study of the same subject made by various authors and limited to a small number of chemicals. -
10-Chemical Resistance
CHEMICAL RESISTANCE GUIDE Tyler Pipe/Soil Pipe Division • 11910 CR 492 • Tyler, TX 75706 • (800) 527-8478 RESISTANCE RATING KEY: E = Excellent G = Good C = Conditional U = Unsatisfactory - = Test Data Not Available AGENT NITRILE NEOPRENE EPOXY STAINLESS STEELDUCTILE/CASTBRONZE IRON ALUMINUM AGENT NITRILE NEOPRENE EPOXY STAINLESS STEELDUCTILE/CASTBRONZE IRON ALUMINUM AGENT NITRILE NEOPRENE EPOXY STAINLESS STEELDUCTILE/CASTBRONZE IRON ALUMINUM AGENT NITRILE NEOPRENE EPOXY STAINLESS STEELDUCTILE/CASTBRONZE IRON ALUMINUM Acetaldehyde U C E E C G G Ammonium Phosphate, Mono-Di-Tri E E E E C C G Acetamide E G E E U U - Ammonium Sulfate E E E G C G G Acetic Acid (Up to 10%, Ammonium Sulfide E E - E U U C 100°F Max.) G E C E U C G Ammonium Thiocyanate E E - G - - - Acetic Acid (10-50%, 100°F Max.) G G G E U C G Amyl Acetate U U E E C C G Acetic Acid, Glacial (100°F Max.) U U G E U C G Amyl Alcohol G G U E C E G Acetic Anhydride U G E E G C G Amyl Borate E E - - - - - Acetone U U U E E E E Amyl Chloride U U E G - E U Acetonitrile G - - - - - - Amyl Chloronaphthalene U U - - - - - Acetaphenone U U - - - - - Amyl Naphthalene U U - - - - - Acrolein, (40%, 120°F) - - - G G G E Aniline U U C E C C C Acrolein, (100%, 200°F) - - - G G G G Aniline Dyes U G - E C C C Acrylic Resin - G - - - - - Aniline Hydrochloride G U - - - - - Acrylonite U U - E C E G Aniline Oil U U E E E E C Acrylonitrile U U E E E E G Animal Fats E G E E E - - Adipic Acid E - - G - - G Animal Oil, (Lard Oil) E G E E E E E Allyl Alcohol, (up to 96%) E E - E E G C Anthraquinone U U