Silver Bromide Nanoparticle/Polymer Composites: Dual Action Tunable Antimicrobial Materials Varun Sambhy, Megan M
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Safety Data Sheet
SAFETY DATA SHEET Revision Date 14-Feb-2020 Revision Number 2 1. Identification Product Name Silver bromide Cat No. : 12110 CAS-No 7785-23-1 Synonyms None Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Company Alfa Aesar Thermo Fisher Scientific Chemicals, Inc. 30 Bond Street Ward Hill, MA 01835-8099 Tel: 800-343-0660 Fax: 800-322-4757 Email: [email protected] www.alfa.com Emergency Telephone Number During normal business hours (Monday-Friday, 8am-7pm EST), call (800) 343-0660. After normal business hours, call Carechem 24 at (866) 928-0789. 2. Hazard(s) identification Classification Classification under 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Based on available data, the classification criteria are not met Label Elements None required Hazards not otherwise classified (HNOC) None identified 3. Composition/Information on Ingredients Component CAS-No Weight % Silver bromide (AgBr) 7785-23-1 99 ______________________________________________________________________________________________ Page 1 / 6 Silver bromide Revision Date 14-Feb-2020 ______________________________________________________________________________________________ 4. First-aid measures Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention. Skin Contact Wash off immediately with soap and plenty of water while removing all contaminated clothes and shoes. Get medical attention. Inhalation Remove from exposure, lie down. Remove to fresh air. Get medical attention. Ingestion Clean mouth with water. Get medical attention. Most important symptoms and No information available. effects Notes to Physician Treat symptomatically 5. Fire-fighting measures Suitable Extinguishing Media Water spray. -

PAPERS READ BEFORE the CHEMICAL SOCIETY. XXII1.-On
View Article Online / Journal Homepage / Table of Contents for this issue 773 PAPERS READ BEFORE THE CHEMICAL SOCIETY. XXII1.-On Tetrabromide of Carbon. No. II. By THOMASBOLAS and CHARLESE. GROVES. IN a former paper* we described several methods for the preparation of the hitherto unknown tetrabromide of carbon, and in the present communication we desire to lay before the Society the results of our more recent experiments. In addition to those methods of obtaining the carbon tetrabromide, which we have already published, the fol- lowing are of interest, either from a theoretical point of view, or as affording advantageous means for the preparation of that substance. Action of Bromine on Carbon Disulphide. Our former statement that? bromine had no action on carbon disul- phide requires some modification, as we find that when it is heated to 180" or 200" for several hundred hours with bromine free from both chlorine and iodine, and the contents of the tubes are neutralised and distilled in the usual way, a liquid is obtained, which consists almost entirely of unaltered carbon disulphide ; but when this is allowed to evaporate spontaneously, a small quantity of a crystalline substance is left, which has the appearance and properties of carbon tetrabromide. The length of time required for this reaction, and the very small relative amount of substance obtained, would, however, render this Published on 01 January 1871. Downloaded by Brown University 25/10/2014 10:39:25. quite inapplicable as a process for the preparation of the tetra- bromide. Action of Bromine on Carbon Disdphide in, presence of Certain Bromides. -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 21-Dec-2020 Version 4.02 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name Silver(I) Bromide Product code 198-00732,190-00731 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research use only restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Short-term (acute) hazardous to the aquatic environment Category 1 Long-term (chronic) hazardous to the aquatic environment Category 1 Pictograms Signal word Warning Hazard statements H400 - Very toxic to aquatic life H410 - Very toxic to aquatic life with long lasting effects Precautionary statements-(Prevention) • Avoid release to the environment Precautionary statements-(Response) • Collect spillage Precautionary statements-(Storage) • Not applicable Precautionary statements-(Disposal) • Dispose of contents/container to an approved waste disposal plant Others Other hazards Not available Section 3: COMPOSITION/INFORMATION ON INGREDIENTS __________________________________________________________________________________________ Page 1 / 6 W01W0119-0073 JGHEEN Silver(I) Bromide Revision Date 21-Dec-2020 __________________________________________________________________________________________ Single Substance or Mixture Substance Formula AgBr Chemical Name Weight-% Molecular weight ENCS ISHL No. CAS RN Silver bromide 97.0 187.77 (1)-2 公表 7785-23-1 Impurities and/or Additives: Not applicable Section 4: FIRST AID MEASURES Inhalation Remove to fresh air. -

Attached Are the MSDS Lists for Various Chemicals Used During the Wet Plate Collodion / Platinum Palladium Courses at Oxbow
Attached are the MSDS lists for various chemicals used during the Wet Plate Collodion / Platinum Palladium courses at OxBow. Many of the materials for this course are highly toxic and therefore thorough communication and understanding about this aspect of wet plate collodion. Safety is a necessity. It is our first priority and concern to having a productive community throughout our courses. Please contact us should you have any questions or concerns, we will be happy to answer. Thank you, Jaclyn Silverman and Robert Clarke-Davis MSDS Lists: Wet-plate collodion * you will be required to use nitrile gloves, there is no exception to this rule. SAFETY DATA SHEET Preparation Date: No data available Revision Date: 6/17/2015 Revision Number: G1 Product identifier Product code: CO120 Product Name: COLLODION, USP Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2 Reproductive toxicity Category 1B Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 1 Flammable liquids Category 1 Label elements Product code: CO120 Product name: COLLODION, USP 1 / 16 Danger Hazard statements Harmful if swallowed Causes skin irritation Causes serious eye irritation May damage fertility or the unborn child May cause respiratory irritation. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Silver Bromide Other means of identification SDS number 2AL Materion Code 2AL CAS number 7785-23-1 Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Materion Advanced Chemicals Inc. Address 407 N 13th Street 1316 W. St. Paul Avenue Milwaukee, WI 53233 United States Telephone 414.212.0257 E-mail [email protected] Contact person Noreen Atkinson Emergency phone number Chemtrec 800.424.9300 2. Hazard(s) identification Physical hazards Not classified. Health hazards Not classified. Environmental hazards Hazardous to the aquatic environment, acute Category 1 hazard Hazardous to the aquatic environment, Category 1 long-term hazard OSHA defined hazards Not classified. Label elements Signal word Warning Hazard statement Very toxic to aquatic life. Very toxic to aquatic life with long lasting effects. Precautionary statement Prevention Avoid release to the environment. Response Collect spillage. Storage Store away from incompatible materials. Disposal Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard(s) not otherwise None known. classified (HNOC) Supplemental information None. 3. Composition/information on ingredients Substances Material name: Silver Bromide SDS US 2AL Version #: 02 Revision date: 01-15-2018 Issue date: 06-05-2015 1 / 6 Chemical name Common name and synonyms CAS number % Silver Bromide 7785-23-1 90 - 100 *Designates that a specific chemical identity and/or percentage of composition has been withheld as a trade secret. 4. First-aid measures Inhalation Move to fresh air. Call a physician if symptoms develop or persist. Skin contact Wash off with soap and water. Get medical attention if irritation develops and persists. -
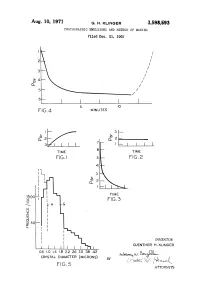
Aug. 10, 1971 G. H. KLINGER 3,598,593 PHOTOGRAPHIC EMUISIONS and METHOD of MAKING Filed Dec
Aug. 10, 1971 G. H. KLINGER 3,598,593 PHOTOGRAPHIC EMUISIONS AND METHOD OF MAKING Filed Dec. 21, 1965 FG4 MINUTES TIME FG.2 8 OO INVENTOR GUENTHER H. KLINGER O 6 .O 1.4 .8 222.2 262.6 3.O 388 4.42 w). (e 38. CRYSTAL DIAMETER (MICRONs) by f 1 ( i I F.G. 5 v. T. ATTORNEYs 3,598,593 United States Patent Office Patented Aug. 10, 1971 1. 2 In the general case of ammonia emulsions, the am 3,598,593 monia can either be added with the silver nitrate solu PHOTOGRAPHC EMULSIONS AND METHOD OF MAKNG tion or it may be added after mixing the silver nitrate and Guenther Harald Kinger, Binghamton, N.Y., assignor to part or all of the halide solution. GAF Corporation, New York, N.Y. 5 According to further aspects of the present invention, Filed Dec. 21, 1965, Ser. No. 515,391 after it was found that it is not essential to maintain a Int, C. G03c 1/02 substantial excess of halide ions, a more detailed in U.S. C. 96-94 8 Claims vestigation on correlation of crystal growth and ion con centrations in the photographic emulsions was under 10 taken. As a result of this investigation it was further found ABSTRACT OF THE DISCLOSURE that it is possible to use only a very small excess of Preparation of gelatin-silver bromide photographic halide ions and still obtain excellent products. It was also emulsions, involving precipitation of the silver bromide by found that even a small excess of silver ions could be admixture of aqueous solutions of a water soluble silver employed during the crystal silver halide grain formation. -

THE MONATOMIC IONS! 1. What Is the Formula for Silver? Ag 2. What Is
Name: ______________________________ THE MONATOMIC IONS! 1. What is the formula for silver? Ag+ 22. What is the formula for cobalt (II)? Co2+ 2. What is the formula for cadmium? Cd2+ 23. What is the formula for chromium (II)? Cr2+ 3. What is the formula for manganese (II)? Mn2+ 24. What is the formula for copper (II)? Cu2+ 4. What is the formula for nickel (II)? Ni2+ 25. What is the formula for tin (IV)? Sn4+ 5. What is the formula for chromous? Cr2+ 26. What is the formula for lead (IV)? Pb4+ 6. What is the formula for zinc? Zn2+ 27. What is the formula for iron (III)? Fe3+ 2+ 2+ 7. What is the formula for cobaltous? Co 28. What is the formula for mercury (I)? Hg2 8. What is the formula for cuprous? Cu+ 29. What is the formula for lead (II)? Pb2+ 9. What is the formula for ferrous? Fe2+ 30. What is the formula for mercury (II)? Hg2+ 2+ 2+ 10. What is the formula for mercurous? Hg2 31. What is the formula for iron (II)? Fe 11. What is the formula for stannous? Sn2+ 32. What is the formula for copper (I)? Cu+ 12. What is the formula for plumbous? Pb2+ 33. What is the formula for tin (II)? Sn2+ 13. What is the formula for chromic? Cr3+ 34. What is the formula for fluoride? F- 14. What is the formula for cobaltic? Co3+ 35. What is the formula for chloride? Cl- 15. What is the formula for cupric? Cu2+ 36. What is the formula for hydride? H- 16. -

SILVER BROMIDE OPTICAL CRYSTAL According to Regulation (EC) No.1907/2006 (REACH) Revision 2016 : Issued 11Th May 2016 1
SAFETY DATA SHEET SILVER BROMIDE OPTICAL CRYSTAL According to Regulation (EC) No.1907/2006 (REACH) Revision 2016 : Issued 11th May 2016 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY 1.1. PRODUCT IDENTIFIERS: Product Name: Silver Bromide Optical Crystal Synonyms, Trade Names: AgBr 1.2. RELEVANT IDENTIFIED USES OF THE SUBSTANCE OR MIXTURE AND USES ADVISED AGAINST Identified Uses: Optical Material for manufacture of Optical Components. 1.3. DETAILS OF THE SUPPLIER OF THE SAFETY DATA SHEET Company: CRYSTRAN LTD, 1 Broom Road Business Park, Poole, Dorset UK BH12 4PA +44 1202 307650 1.4. EMERGENCY TELEPHONE NUMBER Emergency Phone: +44 1202 307650 (Monday to Friday 08:30 to 17:00 GMT) Emergency Action: In the event of a medical enquiry involving this product, please contact your doctor or local hospital accident and emergency department. The attending health professional will be able to contact the National Poisons Information Service. 2. HAZARDS IDENTIFICATION 2.1. CLASSIFICATION OF THE SUBSTANCE OR MIXTURE Harmful by ingestion, inhalation and skin contact. Prolonged exposure may result in argyria, a bluish discolouration of the skin. Irritating to eyes and may irritate skin. Dangerous for the environment. 2.2. LABEL ELEMENTS Signal Word: Warning H290 May be corrosive to metals. H400 Very toxic to aquatic life. Precautionary Statements: P273 Avoid release to the environment. 2.3. OTHER HAZARDS May cause irritation to skin and respiratory tract. 3. COMPOSITION/INFORMATION ON INGREDIENTS 3.1. SUBSTANCES Component Name CAS number % EC number (EINECS) EU index UN number Silver Bromide 7785-23-1 100% 232-076-8 - 3077 4. FIRST AID MEASURES 4.1. -

The Photographic Emulsion; the Silver Ion-Gelatin Equilibrium
RP376 THE PHOTOGRAPHIC EMULSION; THE SILVER ION-GELATIN EQUILIBRIUM By Burt H. Carroll and Donald Hubbard ABSTRACT Electro metric determination of silver ion activity in silver nitrate-gelatin solutions showed strong selective combination of silver ion with gelatin. This decreases with increasing hydrogen ion concentration, but does not vanish on the a,cid side of the isoelectric point. Similar measurements in silver bromide- immonia-gelatin solutions detected no combination of gelatin with the silver- immonia ion. The hydrolysis of silver bromide and chloride in thoroughly washed emulsions was calculated from the electrometric data and verified by direct experiment. CONTENTS Page I. Introduction 811 II. Equilibrium in gelatin-silver nitrate solutions at varying hydrogen ion concentrations 814 1. Apparatus 814 2. Silver ion concentrations 817 3. Discussion 819 III. Equilibrium in silver bromide-ammonia-gelatin solutions 821 IV. The composition of emulsions washed to equilibrium 823 V. Summary 825 I. INTRODUCTION The buffer action of gelatin—its capacity to combine with either acids or bases the change in hydrogen ion concentration | and reduce resulting from the addition of the acid or base to the solution—is well known and important in several types of photographic phenomena. iThe object of this paper is to call attention to a similar capacity of gelatin for combination with silver ions, and some of its photographic | consequences. It should first be pointed out that in the combination of gelatin with acids or bases, the hydrogen or hydroxyl ions are combined in a manner which may under most conditions be considered unique. When, for example, gelatin is added to a solution of hydrochloric acid, the activity of the hydrogen ion as determined by any of the well- known methods is greatly reduced, while that of the chlorine ion is unaffected in dilute solutions. -

Safety Data Sheet According to OSHA HCS Printing Date 09/18/2015 Reviewed on 09/11/2015
Page 1/7 Safety Data Sheet according to OSHA HCS Printing date 09/18/2015 Reviewed on 09/11/2015 1 Identification · Product name · Trade name: Silver bromide (99.9%-Ag) · Item number: 93-4705 · CAS Number: 7785-23-1 · EC number: 232-076-8 · Details of the supplier of the safety data sheet · Manufacturer/Supplier: Strem Chemicals, Inc. 7 Mulliken Way NEWBURYPORT, MA 01950 USA [email protected] · Information department: Technical Department · Emergency telephone number: EMERGENCY: CHEMTREC: + 1 (800) 424-9300 During normal opening times: +1 (978) 499-1600 2 Hazard(s) identification · Classification of the substance or mixture The substance is not classified according to the Globally Harmonized System (GHS). · Label elements · GHS label elements not applicable · Hazard pictograms not applicable · Signal word not applicable · Hazard statements not applicable · Precautionary statements P262 Do not get in eyes, on skin, or on clothing. P280 Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P403+P233 Store in a well-ventilated place. Keep container tightly closed. P410 Protect from sunlight. P501 Dispose of contents/container in accordance with local/regional/national/international regulations. · Classification system: · NFPA ratings (scale 0 - 4) Health = 0 0 Fire = 0 0 0 Reactivity = 0 · HMIS-ratings (scale 0 - 4) HEALTH 0 Health = 0 FIRE 0 Fire = 0 REACTIVITY 0 Reactivity = 0 · Other hazards · Results of PBT and vPvB assessment · PBT: Not applicable. (Contd. on page 2) USA 39.5.0 Page 2/7 Safety Data Sheet according to OSHA HCS Printing date 09/18/2015 Reviewed on 09/11/2015 Trade name: Silver bromide (99.9%-Ag) (Contd. -

Safety Data Sheet
Safety Data Sheet Page 1/4 per OSHA HazCom 2012 Printing date 11/24/2015 Reviewed on 04/18/2012 1 Identification Product identifier Product name: Silver bromide Stock number: 12110 CAS Number: 7785-23-1 EC number: 232-076-8 Relevant identified uses of the substance or mixture and uses advised against. Identified use: SU24 Scientific research and development Details of the supplier of the safety data sheet Manufacturer/Supplier: Alfa Aesar Thermo Fisher Scientific Chemicals, Inc. 30 Bond Street Ward Hill, MA 01835-8099 Tel: 800-343-0660 Fax: 800-322-4757 Email: [email protected] www.alfa.com Information Department: Health, Safety and Environmental Department Emergency telephone number: During normal business hours (Monday-Friday, 8am-7pm EST), call (800) 343-0660. After normal business hours, call Carechem 24 at (866) 928-0789. 2 Hazard(s) identification Classification of the substance or mixture in accordance with 29 CFR 1910 (OSHA HCS) The substance is not classified according to the Globally Harmonized System (GHS). Hazards not otherwise classified No information known. Label elements GHS label elements Not applicable Hazard pictograms Not applicable Signal word Not applicable Hazard statements Not applicable WHMIS classification Not controlled Classification system HMIS ratings (scale 0-4) (Hazardous Materials Identification System) HEALTH 1 Health (acute effects) = 1 FIRE 1 Flammability = 1 REACTIVITY 1 Physical Hazard = 1 Other hazards Results of PBT and vPvB assessment PBT: Not applicable. vPvB: Not applicable. 3 Composition/information on ingredients Chemical characterization: Substances CAS# Description: 7785-23-1 Silver bromide Identification number(s): EC number: 232-076-8 4 First-aid measures Description of first aid measures After inhalation Supply fresh air.