Patterson, A. 2012. Breeding and Foraging Ecology of Caspian Terns
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Breeding and Foraging Ecology of Caspian Terns Nesting on Artificial Islands in the Upper Klamath Basin, California
AN ABSTRACT OF THE THESIS OF Allison Patterson for the degree of Master of Science in Wildlife Science presented on November 13, 2012 Title: Breeding and Foraging Ecology of Caspian Terns Nesting on Artificial Islands in the Upper Klamath Basin, California Abstract approved: Daniel D. Roby Availability of suitable nesting habitat that is free of nest predators and provides access to adequate prey resources within commuting distance is a major factor limiting seabird populations. Caspian terns (Hydroprogne caspia ) in western North America have shifted their breeding habitat from naturally occurring habitats in interior wetlands, lakes, and rivers to primarily human-created habitats in coastal bays and estuaries. This shift has brought Caspian terns into conflict with fisheries of conservation concern, in particular anadromous salmonids. Prior to the 2010 breeding season, three artificial islands were built in the Klamath Basin National Wildlife Refuge (NWR) Complex as alternative nesting habitat for Caspian terns currently nesting at the world’s largest colony for the species, near the mouth of the Columbia River, Oregon. I investigated the efficacy of habitat creation (island building) and social attraction (decoys and recorded vocalizations) for establishing new breeding colonies in the Upper Klamath Basin, California. In 2010, approximately 258 pairs of Caspian terns attempted to nest on the new islands and raised an average of 0.65 fledglings/breeding pair; in 2011, 222 pairs attempted to nest and raised an average of 0.11 fledglings/breeding pair. Competition with California and ring-billed gulls ( Larus californicus and L. delawarensis ) for nesting space, gull predation on Caspian tern eggs and chicks, low water levels, and depredation by great horned owls (Bubo virginianus ) were the primary factors limiting colony development and productivity, especially in 2011. -

MVUM) Were Made by the Responsible Official Pursuant to 36 !
*# ! ! ! ! ! ! ! *# ! ! ! ! ! ! ! ! ! *# ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! *# ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! *# ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 3 ! ! ! ! ! ! ! ! ! 1 ! ! ! ! ! 4 ! ! 3 ! ! ! 5 ! 6 ! ! ! ! ! ! ! 3 ! ! ! ! 1 2 ! ! ! 7 ! 4 ! ! ! ! ! ! 3 ! ! 3 ! 2 ! ! ! ! 2 7 ! ! ! ! ! ! ! ! 3 ! ! ! ! 2 ! 1 0 1 1 0 ! ! ! ! ! 5 ! 2 ! ! ! ! 7 3 ! 9 ! 1 ! 7 5 ! 7 ! ! ! ! ! ! ! 0.3 570 1 ! ! ! ! ! 3.9 0 0.3 ! 0 2 3 ! 0 ! 7 ! 1 3 ! 0 ! 6 ! ! ! ! 1 ! ! ! 0 ! 4 ! ! 3 ! ! ! 0 ! ! 7 ! 3462231 ! ! 0.3 ! ! ! ! 3 ! 0.6 5! ! 2 ! ! ! 3 ! ! ! ! ! ! ! ! ! ! 3 0.5 ! ! ! ! 1 2 0.9 ! ! 0 ! ! ! 5 ! 3 ! ! ! 0 ! 4 4 ! ! ! ! ! 2 ! 2 ! 1!7 ! 2700 7 ! ! ! ! ! 3300134 4 ! ! ! 7 ! ! ! ! ! ! 3 1 3300135 ! ! 6 ! 0 7 ! 2 ! ! 0 ! ! 3 0 ! 7 !! ! 0.7 ! ! ! ! ! 2 ! ! ! MARSTER ! ! 1 ! ! 0.5 0 ! ! ! 0 ! ! 1.3 2 ! ! ! ! ! ! ! 2 ! 332 ! ! 3118 ! ! ! 5 5 ! 0 ! 7 # # ! ! ! * * *# ! ! ! ! 7 ! ! 0.8 # ! * 1 1 5 ! ! ! ! ! 0 ! SPRING ! ! ! ! ! ! 7 0 3 ! ! ! ! 3 ! ! ! 3 0.8 3 ! ! ! ! 3 0 ! ! ! 4 ! 0 4 2 ! 0.7 ! 6 0 ! ! ! ! 4 6 ! 8 3 3 ! ! 0 ! ! ! 2 ! *# 5 6 7 ! 0 ! 2 ! 0 0 ! 4 ! ! ! 1 ! 0 ! 0 ! 3 ! 3 ! 0 ! ! 5 3 ! 3 ! 3 2 0 ! 3 3462025 ! ! 1 ! ! ! ! 0 5 ! 3 ! ! ! 4 ! ! ! ! 0 ! 4 ! 4 1 ! 0 ! ! 0 ! ! 1.6 5 ! ! ! 6 ! " ! ! 0 ! ! 0 5 0 ! 4 ! ! 9 1 ! ! 2 ! 1 ! ! 4 ! 3 ! 3 ! ! ! ! 0 ! ! 0.4 ! ! ! 7 0 # 5 ! * 2 # 0 Black Butte ! 3 1 ! * 7 ! ! ! ! ! 0 8 4 1 ! ! ! 2 4 2 # 1.1 ! 2.5 ! ! * ! ! 0 ! ! 0 ! 6 1.1 ! 2 ! # * ! ! 4 # ! 6 # ! * * ! 2 3 ! ! 3462200 # *# * ! ! 1.4 ! ! 8 5 ! ! ! ! ! ! ! ! ! ! 1 ! R. 12 E. R. 13 E. 3.7 R. 14 E. R. 15 E. ! R. 16 E. R. 17 E. R. -
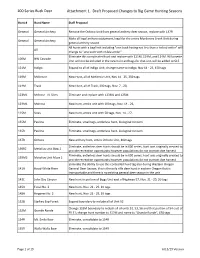
100 Series Buck Deer Attachment 1. Draft Proposed Changes to Big Game Hunting Seasons
100 Series Buck Deer Attachment 1. Draft Proposed Changes to Big Game Hunting Seasons Hunt # Hunt Name Staff Proposal General General Archery Remove the Ochoco Unit from general archery deer season, replace with 137R Make all legal archery equipment, legal for the entire Murderers Creek Unit during General General Archery general archery season All hunts with a bag limit including "one buck having not less than a forked antler" will All change to "one buck with visible antler" Eliminate this complicated hunt and replace with 121M, 119M, and 114M. Willamette 100M NW Cascade Unit will not be included in the new hunt and tags for that unit will be added to 615 121M Indigo Expand to all of Indigo Unit, change name to Indigo, Nov 14 - 25, 150 tags 119M McKenzie New Hunt, all of McKenzie Unit, Nov 14 - 25, 350 tags 114M Trask New Hunt, all of Trask, 200 tags, Nov. 7 - 20, 123M1 Melrose - N. Sixes Eliminate and replace with 123M1 and 125M 123M1 Melrose New hunt, entire unit with 100 tags, Nov. 14 - 22, 125M Sixes New hunt, entire unit with 50 tags, Nov. 14 - 27, 135M Paulina Eliminate, small tags, antlerless hunt, biological concern 135R Paulina Eliminate, small tags, antlerless hunt, biological concern 137R Ochoco New archery hunt, entire Ochoco Unit, 400 tags Eliminate, antlerless deer hunts should be in 600 series, hunt was originally created to 139R2 Metolius Unit Bow 2 provide recreation opportunity however populations do not warrant doe harvest Eliminate, antlerless deer hunts should be in 600 series, hunt was originally created to 139M2 Metolius Unit Muzz 2 provide recreation opportunity however populations do not warrant doe harvest Eliminate the ability to use this controlled hunt tag also during Western Oregon 141A Hood-White River General Deer Season, this is the only rifle deer hunt in eastern Oregon that is transportable and there is no existing general deer season in the unit 143C John Day Canyon New hunt in portion of Biggs Unit east of Highway 97, Nov. -

RAPTOR RESEARCH REPORTS a Publication of the Raptor Research Foundation) Inc
(ISBN 0-935868-43-7) RAPTOR RESEARCH REPORTS A Publication of The Raptor Research Foundation) Inc. RAPTOR HABITAT MANAGEMENT UNDER THE U.S. BUREAU OF LAND MANAGEMENT MULTIPLE-USE MANDATE April 1989 No.8 RAPTOR RESEARCH REPORTS F.stablisbed 1971 EDITOR Jimmie R. Parrish The Raptor Research Foundation. Inc. Depamneot of Zoology, 159 Widtsoe Building Brigham Young UniveiSity Provo, Utah 84602 Consulting EditoiS forVolume8 Jeffrey L. Lincer RicbardJ. aam: W. Grainger Hunt Eco-analysts, Inc. Depamnent of Biology BioSystems Analysis, Inc. 4718 Dunn Drive York College of Pennsylvania 303 Potrero Street, Suite 29-203 Sarasota, Florida 33583 Yod:, Pennsylvania 17403-3426 Santa Cmz, California 95060 The Raptor Research Foundation, Inc., was formed in 1966 by individuals who recognized the impact of human activities on raptors and other forms of wildlife. Information provides the key to understanding the life history and ecology of raptor species. The purpose of The Raptor Research Foundation, Inc., is "to stimulate the dissemination of information concerning raptorial birds among interested persons worldwide and to promote a better public understanding and appreciation of the value of birds of prey" (Article I, Section 2, By-Laws of the Raptor Research Foundation, Inc.). Raptor Research Reports was first published in 1971. Articles too lengthy for inclusion in the Journal of Raptor Research are published as Rap tor Research Reports as determined by the Editor and Board of Directors. No set schedule for publication is prescribed. Individual volumes are published based on availability of material and following completion of financial arrangements with the Editorial Office. Generally, manuscrlets considered for publication in Raptor Research Reports exceed 50 pages in length, including text, illustrations, tables, and literature cited. -

Terrestrial Wildlife Species Habitat Descriptions
TERRESTRIAL WILDLIFE SPECIES HABITAT DESCRIPTIONS Fremont-Winema National Forest Fremont Land and Resource Management Plan Eastside of the Forest - Bly, Lakeview, Paisley, and Silver Lake Districts JULY 17, 2017 PREPARED BY Cheran Cavanaugh – Eastside Wildlife Biologist and Isaac Gansberg – Eastside Assistant Wildlife Biologist Table of Contents Introduction .................................................................................................................................... 3 Mammals ........................................................................................................................................ 5 American marten ........................................................................................................................ 5 Canada lynx ................................................................................................................................. 8 Gray Wolf .................................................................................................................................... 9 Fringed myotis .......................................................................................................................... 11 Mule Deer ................................................................................................................................. 12 Pacific Fisher – West Coast Distinct Population Segment (DPS) .............................................. 15 Pallid bat .................................................................................................................................. -

Rock Features of the Upper Klamath Basin: an Integrated Approach to Identification
Central Washington University ScholarWorks@CWU All Master's Theses Master's Theses Winter 2018 Rock Features of the Upper Klamath Basin: An Integrated Approach to Identification Roman Jakien Central Washington University, [email protected] Follow this and additional works at: https://digitalcommons.cwu.edu/etd Part of the Archaeological Anthropology Commons Recommended Citation Jakien, Roman, "Rock Features of the Upper Klamath Basin: An Integrated Approach to Identification" (2018). All Master's Theses. 892. https://digitalcommons.cwu.edu/etd/892 This Thesis is brought to you for free and open access by the Master's Theses at ScholarWorks@CWU. It has been accepted for inclusion in All Master's Theses by an authorized administrator of ScholarWorks@CWU. For more information, please contact [email protected]. ROCK FEATURES OF THE UPPER KLAMATH BASIN: AN INTEGRATED APPROACH TO IDENTIFICATION ____________________________________ A Thesis Presented to The Graduate Faculty Central Washington University ____________________________________ In Partial Fulfillment of the Requirements for the Degree Master of Science Cultural and Environmental Resource Management ____________________________________ by Roman Stanley Jakien March 2018 CENTRAL WASHINGTON UNIVERSITY Graduate Studies We hereby approve the thesis of Roman Stanley Jakien Candidate for the degree of Master of Science APPROVED FOR THE GRADUATE FACULTY ______________ __________________________________________ Dr. Patrick Lubinski, Committee Chair ______________ __________________________________________ Dr. Steve Hackenberger ______________ __________________________________________ Sara Boyko ______________ __________________________________________ Dean of Graduate Studies ii ABSTRACT ROCK FEATURES OF THE UPPER KLAMATH BASIN: AN INTEGRATED APPROACH TO IDENTIFICATION by Roman Stanley Jakien March 2018 Rock features are human-made rock structures, often created by native peoples in the past and currently recorded as archaeological sites. -

The Gerber Family Legacy on Public Lands (Sources Not Referenced in Text)
The Gerber Family Legacy on Public Lands (sources not referenced in text) Brooke Brown Klamath Falls Resource Area Lakeview District OR/WA BLM Setting The Gerber Block is located on Bureau of Land Management (BLM) lands in southern Oregon, and managed by the Klamath Falls Resource Area out of the Lakeview District. The ‘Gerber Block’ encompasses 8 different legal descriptions comprised of townships and ranges. The west side of what is known today as the ‘Gerber Block’ was described in 1868 and 1871, by early cadastral surveyors as an environment that produced grasses with prairie lands surrounded by timber. The notes also describe areas that contain numerous springs, and locations ‘… well adapted to grazing,’ with areas appropriate for agriculture. Environmentally, they note much of the prairie locales seem to be marshy and wet in the spring. The south-central part of the Gerber Block is noted in the 1872 and 1875 cadastral notes as being comprised of high table lands with juniper and grasses, and comment on the lands as being valuable grazing pasture. The eastern side of the Gerber Block was observed in 1872 and 1875 as having some lands appropriate for agricultural development, and again, took note of the high table mesas of this area that would be beneficial for pasture, along with mixed timber and grasses. Cadastral notes for the north-central portion of the Gerber Block dating from 1952 document roads through the area and describe the dominant feature being the Gerber Reservoir that was constructed for flood control and irrigation needs in the region. The area is still noted to be rocky prairie with pockets of heavy timber. -

Oregon Guest Editorial
p I i L- w ' si: s D I OREGON GUEST EDITORIAL WILDLIFE Editor's Note: In recent months, especially since the setting of the 1980 ocear. have been a number of accusations and implications MAY i 980 salmon seasons, there Volume 35, No. 5 concerning the Director of the Department of Fish and Wildlife, Jack Donald- son. Not a few of the comments have been based on misinformation and a lack of understanding of certain procedures and responsibilities. OREGON FISH AND WILDLIFE COMMISSION At the April 4 Fish and Wildlife Commission meeting an editorial suggesting the Director should resign was discussed by the Commissioners. As a result Herbert Lundy, Chairman ......... Lake Oswego the ensuing discussion the following letter was written Jack Steiwer, Vice Chairman ............. Fossil of that editorial and Since we feel it succinctly addresses Donald Barth ...................... Newport by Commission Chairman Herb Lundy. John Boyer ..................... Beilfountain a number of the most heard allegations of recent months, we pass it along Allan Kelly ........................ Portland for your information. Kenneth Klarquist .................. Portland R.E.S. Fred Phillips ......................... Baker the Editor JOHN R. DONALDSON, Director To Bay Reporter Coos Bay, OR This is to inform you and your readers, in response to your recent editorial Oregon Wildlife (ISSN 0094-7113) is published monthly by the Oregon State Department of Fish suggesting that John R. Donaldson should resign as Director of the Oregon and Wildlife, Portland, Oregon. Volumes i through Fish and Wildlife Department, that the Fish and Wildlife Commission, in 28 were entitled Oregon Game Commission Bulletin. Oregon Wildlife is circulated free of charge with an unanimous vote on April 3, 1980, expressed complete confidence in Director second class postage paid at Portland, Oregon. -

Number 3 -- Spring 1987
I a PARK SCUENCE A RESOURCE MANAGEMENT BULLETIN NATIONAL PARK SERVICE U.S. DEPARTMENT OF THE INTERIOR VOLUME7 - NUMBER3 SPRING1987 q PARK SPRING 1987 A report to park managers of recent and on- SCIENCE going research in parks with emphasis on its NATIONAL PARK SERVICE implications for planning and management In This Issue Page Meet the New AD Meet the New AD .......................... 2 Dr. F Eugene Hester has been appointed Associate How Urban Development Near Director for Natural Resources for the Nattonal Park Parks is Affecting Streams .................. .3 Service. Before joining the Service, he was Deputy Director of the U.S. Ftsh and Wildlife Service (19% Soleduck Revegetation Project .............. .4 1967). Hester began his caleer in 1959 as Assistant Using 35mm Color Infrared Professor of Zoology at Norih Carolina State Univer- Slides to Map Vegetation .................... 5 sity In 1963, he left this position to become the leader Letters to the edltor ........................ 6 of the North Carolina Cooperative Fishery Research Unit of the USFWS. He became Chief of the Division MAB Notes ............................... 7 of Fishery Research in 1971 and later was made As- BookReview ............................. sociate Director for Research (1974-1981). Among the Tom Lucke ............................... 7 nurnelous awards Hester has received for his service The Boundary Approach to the Analysis with the Federal Government, are the Department of of Conservation of Nature Reserves .......... (8 the Interior Meritorious Service Award (1981) and a Presidential DIstinguished Rank Award (1982). Computer Corner ......................... .9 He has a B.S. from North Carolina State University, Conservation Biology Group an M.S. in wildlife biology from North Carolina State, Exhibits ‘Elastic’ Response ................ -
Lost River and Shortnose Sucker
U.S. Fish & Wildlife Service Lost River & Shortnose Sucker (DelƟstes luxatus & Chasmistes brevirostris) Both the Lost River sucker (C’waam) Historical InformaƟon: Early records Because suckers were historically very and shortnose sucker (Qapdo) are indicate these fish were once wide- abundant, they were a major food large, long-lived freshwater fish found spread and abundant in the upper Kla- source for the Klamath and Modoc In- exclusively in the Klamath Basin. Both math Basin of Oregon and California. dians and local selers in the late fish were once a valuable source of This area historically contained over 1800's. Canneries were established food and oil and are considered sacred 350,000 acres of wet-lands and flood- along the Lost River to process suckers by the Basin’s Nave American tribes. plains. These areas protected sucker into oil, dried fish, and other products. These fish were federally listed as en- habitat by controlling erosion, recy- dangered in 1988. A recovery plan was cling organic and inorganic nutrients, published in 1993. and maintaining water quality. Agricultural development and associat- decline, it is believed that the com- through the degradaon of suitable ed water and land use changes in the bined effects of the construcon of breeding habitat. basin have contributed to the signifi- dams, the draining or dredging of At the me the suckers were listed as cant loss of wetland habitat and a sig- lakes, and other alteraons of natural endangered, it was noted that there nificant decline in sucker populaons. stream flow have reduced the repro- had been no significant addion of Although overharvesng and polluon ducve success of shortnose and Lost young into the populaon in 18 years. -

Common Plants of the Upper Klamath Basin
Common Plants of the Upper Klamath Basin Technical Layout & Design .........Michael Calonje Editor ................................................Sarah Malaby Plant Descriptions & Text ..............Molly Juillerat, Ron Larson, Sarah Malaby, Jeanne Skalka. Photography ...........Michael Calonje, Ron Larson, Sarah Malaby, Terry Spivey. A Special acknowledgement to Klamath County Commissioners Al Switzer, John Elliott and Bill Brown for providing funding for publication costs through PL 06-393 Title III “Secure Rural Schools and Community Self-Determination Act of 2000” Oregon Native Plant Society - Klamath Basin Chapter Rabe Consulting 2007 CONTENTS Introduction ...................................................................................3 Overview ........................................................................................3 Habitats ..........................................................................................4 Plant Exploration in the Upper Klamath Basin .........................6 Growing Native Plants ..................................................................6 Species Groups ..............................................................................7 Ferns and Horsetails ..................................................................7 Conifers .....................................................................................8 Flowering Plants: Flowers, Hardwood Trees, and Shrubs .......9 Flowering Plants: Grasses and Grass-like Plants ...................9 Lichens, Bryophytes, and Blue-green -

The Distribution of Geologic and Artifact Obsidian from the Silver Lake/Sycan Marsh
AN ABSTRACT OF THE THESIS OF Jennifer J. Thatcher for the degree of Master of Arts in Interdisciplinary Studies in Anthropology. Anthropology, and Geography presented on December 8. 2000. Title: The Distribution of Geologic and Artifact Obsidian from the Silver Lake/Sycan Marsh Geochemical Source Group. South-Central Oregon. Redacted for Privacy Abstract approved: J. Roth Geochemical characterization methods are commonly used in the reconstruction of prehistoric raw material use and procurement systems. Trace element studies of lithic source material and artifacts, specifically those made of obsidian, can reveal important information about the environmental and cultural factors which influence the prehistoric distribution of raw material. The current investigation uses geochemical characterization methods and data to document and evaluate the distribution of geologic and artifact obsidian that originates from the Silver Lake/Sycan Marsh (SL/SM) obsidian source. This large and prehistorically significant source is located in western Lake County, Oregon. Few source descriptions or artifact distribution studies exist for SL/SM obsidian. However, over the past decade, a significant increase in the use of geochemical characterization methods has generated a wealth of data for Oregon obsidian sources. This thesis synthesizes the results of the geochemical characterization analysis of 392 geologic obsidian specimens collected from the SL/SM source area and 1,938 SL/SM obsidian artifacts recovered from over 200 archaeological sites in Oregon, Washington and California. The artifact analytical data were derived from previously characterized artifact collections compiled and archived in an extensive database. A subset of artifacts were characterized for the purpose of this study. Based on the results of geochemical analysis of the geologic material, two distinct source boundaries are defined for the SL/SM geochemical source.