Ecology of the Sontecomapan Lagoon, Veracruz
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Zootaxa 3266: 41–52 (2012) ISSN 1175-5326 (Print Edition) Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (Online Edition)
Zootaxa 3266: 41–52 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) Thalasseleotrididae, new family of marine gobioid fishes from New Zealand and temperate Australia, with a revised definition of its sister taxon, the Gobiidae (Teleostei: Acanthomorpha) ANTHONY C. GILL1,2 & RANDALL D. MOOI3,4 1Macleay Museum and School of Biological Sciences, A12 – Macleay Building, The University of Sydney, New South Wales 2006, Australia. E-mail: [email protected] 2Ichthyology, Australian Museum, 6 College Street, Sydney, New South Wales 2010, Australia 3The Manitoba Museum, 190 Rupert Ave., Winnipeg MB, R3B 0N2 Canada. E-mail: [email protected] 4Department of Biological Sciences, 212B Biological Sciences Bldg., University of Manitoba, Winnipeg MB, R3T 2N2 Canada Abstract Thalasseleotrididae n. fam. is erected to include two marine genera, Thalasseleotris Hoese & Larson from temperate Aus- tralia and New Zealand, and Grahamichthys Whitley from New Zealand. Both had been previously classified in the family Eleotrididae. The Thalasseleotrididae is demonstrably monophyletic on the basis of a single synapomorphy: membrane connecting the hyoid arch to ceratobranchial 1 broad, extending most of the length of ceratobranchial 1 (= first gill slit restricted or closed). The family represents the sister group of a newly diagnosed Gobiidae on the basis of five synapo- morphies: interhyal with cup-shaped lateral structure for articulation with preopercle; laterally directed posterior process on the posterior ceratohyal supporting the interhyal; pharyngobranchial 4 absent; dorsal postcleithrum absent; urohyal without ventral shelf. The Gobiidae is defined by three synapomorphies: five branchiostegal rays; expanded and medially- placed ventral process on ceratobranchial 5; dorsal hemitrich of pelvic-fin rays with complex proximal head. -

Pacific Plate Biogeography, with Special Reference to Shorefishes
Pacific Plate Biogeography, with Special Reference to Shorefishes VICTOR G. SPRINGER m SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 367 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3
ACTA ICHTHYOLOGICA ET PISCATORIA (2018) 48 (4): 341–362 DOI: 10.3750/AIEP/02451 AN UPDATED CHECKLIST AND CHARACTERISATION OF THE ICHTHYOFAUNA (ELASMOBRANCHII AND ACTINOPTERYGII) OF THE LAGUNA DE TAMIAHUA, VERACRUZ, MEXICO Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3 1 Posgrado en Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad de México 2 Instituto Nacional de Pesca y Acuacultura, SAGARPA, Ciudad de México 3 Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México Raz-Guzmán A., Huidobro L., Padilla V. 2018. An updated checklist and characterisation of the ichthyofauna (Elasmobranchii and Actinopterygii) of the Laguna de Tamiahua, Veracruz, Mexico. Acta Ichthyol. Piscat. 48 (4): 341–362. Background. Laguna de Tamiahua is ecologically and economically important as a nursery area that favours the recruitment of species that sustain traditional fisheries. It has been studied previously, though not throughout its whole area, and considering the variety of habitats that sustain these fisheries, as well as an increase in population growth that impacts the system. The objectives of this study were to present an updated list of fish species, data on special status, new records, commercial importance, dominance, density, ecotic position, and the spatial and temporal distribution of species in the lagoon, together with a comparison of Tamiahua with 14 other Gulf of Mexico lagoons. Materials and methods. Fish were collected in August and December 1996 with a Renfro beam net and an otter trawl from different habitats throughout the lagoon. The species were identified, classified in relation to special status, new records, commercial importance, density, dominance, ecotic position, and spatial distribution patterns. -

Anarchist Movements in Tampico & the Huaste
UNIVERSITY OF CALIFORNIA, SAN DIEGO Peripheries of Power, Centers of Resistance: Anarchist Movements in Tampico & the Huasteca Region, 1910-1945 A Thesis submitted in partial satisfaction of the requirements for the degree Master of Arts in Latin American Studies (History) by Kevan Antonio Aguilar Committee in Charge: Professor Christine Hunefeldt, Co-Chair Professor Michael Monteon, Co-Chair Professor Max Parra Professor Eric Van Young 2014 The Thesis of Kevan Antonio Aguilar is approved and it is acceptable in quality and form for publication on microfilm and electronically: Co-Chair Co-Chair University of California, San Diego 2014 iii DEDICATION: For my grandfather, Teodoro Aguilar, who taught me to love history and to remember where I came from. iv TABLE OF CONTENTS Signature Page……………………………………………………………..…………..…iii Dedication……………………………………………………………………………...…iv Table of Contents………………………………………………………………………….v List of Figures………………………………………………………………………….…vi Acknowledgements………………………………………………………………………vii Abstract of the Thesis…………………………………………………………………….xi Introduction……………………………………………………………………………......1 Chapter 1: Geography & Peripheral Anarchism in the Huasteca Region, 1860-1917…………………………………………………………….10 Chapter 2: Anarchist Responses to Post-Revolutionary State Formations, 1918-1930…………………………………………………………….60 Chapter 3: Crisis & the Networks of Revolution: Regional Shifts towards International Solidarity Movements, 1931-1945………………95 Conclusion………………………………………………………………………….......126 Bibliography……………………………………………………………………………129 v LIST -

A Dissertation Entitled Evolution, Systematics
A Dissertation Entitled Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) By Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) ____________________________________ Adviser: Dr. Carol A. Stepien ____________________________________ Committee Member: Dr. Christine M. Mayer ____________________________________ Committee Member: Dr. Elliot J. Tramer ____________________________________ Committee Member: Dr. David J. Jude ____________________________________ Committee Member: Dr. Juan L. Bouzat ____________________________________ College of Graduate Studies The University of Toledo December 2009 Copyright © 2009 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. _______________________________________________________________________ An Abstract of Evolution, systematics, and phylogeography of Ponto-Caspian gobies (Benthophilinae: Gobiidae: Teleostei) Matthew E. Neilson Submitted as partial fulfillment of the requirements for The Doctor of Philosophy Degree in Biology (Ecology) The University of Toledo December 2009 The study of biodiversity, at multiple hierarchical levels, provides insight into the evolutionary history of taxa and provides a framework for understanding patterns in ecology. This is especially poignant in invasion biology, where the prevalence of invasiveness in certain taxonomic groups could -

A Checklist of North American Odonata
A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution Dennis R. Paulson and Sidney W. Dunkle 2009 Edition (updated 14 April 2009) A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution 2009 Edition (updated 14 April 2009) Dennis R. Paulson1 and Sidney W. Dunkle2 Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009. Copyright © 2009 Dennis R. Paulson and Sidney W. Dunkle 2009 edition published by Jim Johnson Cover photo: Tramea carolina (Carolina Saddlebags), Cabin Lake, Aiken Co., South Carolina, 13 May 2008, Dennis Paulson. 1 1724 NE 98 Street, Seattle, WA 98115 2 8030 Lakeside Parkway, Apt. 8208, Tucson, AZ 85730 ABSTRACT The checklist includes all 457 species of North American Odonata considered valid at this time. For each species the original citation, English name, type locality, etymology of both scientific and English names, and approxi- mate distribution are given. Literature citations for original descriptions of all species are given in the appended list of references. INTRODUCTION Before the first edition of this checklist there was no re- Table 1. The families of North American Odonata, cent checklist of North American Odonata. Muttkows- with number of species. ki (1910) and Needham and Heywood (1929) are long out of date. The Zygoptera and Anisoptera were cov- Family Genera Species ered by Westfall and May (2006) and Needham, West- fall, and May (2000), respectively, but some changes Calopterygidae 2 8 in nomenclature have been made subsequently. Davies Lestidae 2 19 and Tobin (1984, 1985) listed the world odonate fauna Coenagrionidae 15 103 but did not include type localities or details of distri- Platystictidae 1 1 bution. -

TAKE TOWN on PANUCO RIVER REVENUE BILL by Senate Leaders
RIO GRANDE DELTA EXPOSITION-BROWNSVILLE-FEBRUARY 25,26, 27,28, 29, MARCH 1 j Pmpiat Machinery ! PIPE FITTINGS ... _ .. ; Many «aHoada In atock to fill F»irl»nki Mon* 1 % to It h. p. o,„ ««* I. tb. b..t Oil j | and Atlaa Engiow ^|] ,iHI| «|ia|*« and kind*. Wa Pa no pa. a. fai atock. Cantrifncal B are headquarter W. H. Potegaat Ca x j , u _ ““L'ZL?*" -—- Today s News in Today’s Paper Fall leased wire Associated Press news service -—- — ■■■ ii ■■■■■■■■ ■ .—.i ■■ji.j ,, —.... .--i VOL. XXX, Xo. 233 BROWNSVILLE, TEXAS, FRIDAY FEBRUARY 22,1924 SIX PAGES TODAY ESTABLISHED 1893 .'J AFTERNOON, "" * 1 1 * ■■ — — ■ — ■ —' -- —1 1 — ■■ — ---r— -■■■■■ mj-u-un-n.^ ‘‘ruwu^r r-n-‘-^ ->f-ij-i_t-xj-u-<j~,/-u-i -u-J-.-n-n-^- -'------------------ --------- --- I -- --- Commander States REBEL FORCES Legion WASHINGTON Abandonment Mellon Juggled Figures Where he wearied eye Whom envy dared not hate. paugherty may repose TAKE TOWN ON When gazing on the great. Bequeathed the name of Senate Leaders Cause to Defeat Soldier Bonus When* neither guilty glory glows Washington by --—-—---—-— Nor despicable state? To make men blush there wa» the republican party." John II. i.hiintt, Yes. fine the first, the last, the best. but one. States That Big Business is The t'incinnntus of the Lord PANUCO RIVER national commander «»f the Anieri<nn West, Byron of Tense Situation / its l.cgion. declared b*>re today in nn ud- Spending War Profits This morning, as wr listened to the cannon in Port Brown (looming its Today dti* s i « Aiiieri-au Legion represent!! customary salute in honor of the country's greatest hero, we thought. -

FLORIDA WEST COAST BROMELIAD SOCIETY 1954-2014 Celebrating 60 Years of Bromeliads Floridabromeliads.Org
FLORIDA WEST COAST BROMELIAD SOCIETY 1954-2014 Celebrating 60 Years of Bromeliads floridabromeliads.org July 2014 Newsletter NEXT MEETING Date & Time: Location: Tuesday, July 1 Good Samaritan Church Doors open at 7 pm; meeting starts at 7:30 6085 Park Boulevard Pinellas Park, Florida 33781 Program Dennis Cathcart’s presentation, New Explorations and Discoveries in Brazil--Part I, is based on the recent five-week trip he and his wife Linda took to Brazil that took them through four of Brazil’s 26 states. Their objectives were to find Dyckias and other terrestrial bromeliads and visit some of the bromeliad colonies they had known from past visits to Brazil. Along the way they found the Dyckias and so much more—varied scenery, diverse biological zones and climates, wildlife, insects, and plants. Hopefully the ‘Part I’ in the title means we can look forward to additional chapters in their 2014 Brazil tour story. Dennis and Linda have owned and operated Tropiflora Nursery in Sarasota, Florida, since 1976. The nursery specializes in rare and exotic plants, primarily bromeliads along with orchids, cycads, caudiciforms, succulents, ferns, palms and more. It is one of the largest collector-oriented exotic plant nurseries in the United States and has over 200,000 square feet of greenhouses. Many of the plants in their collection were collected by them as seeds and cuttings on their more than100 collecting expeditions made in over 35 years and in 27 countries. Plant Sales The speaker will be the sole plant vendor for this meeting and there will be no member plant sales. -

Neoneura, Species, Neoneura Jurzitzai
Odonatologica 28(4): 343-375 December I, 1999 The genus Neoneura, with keys and description of a new species, Neoneurajurzitzai spec. nov. (Zygoptera: Protoneuridae) R.W. Garrison Research Associate, Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007, United States Received April 10, 1999 / Reviewed and Accepted May 11, 1999 A all both synopsis of 23 spp. includes keys to sexes, based primarily on caudal appendage morphology in males and morphology of the hind lobe of the prothorax in females, diagnoses, distributional notes and diagnostic illustrations. N. jurzitzai sp.n. (holotype 6: Brazil, Santa Catarina state, Nova Teutonia, 5 XI-I942, in UMMZ) is described, and the 6 and 9 ofN. rufithorax Selys are described based on specimens from Peru. INTRODUCTION include The genus Neoneura was first proposed by SELYS (1860) to two new species, N. rubriventris and N. bilinearis. Later, SELYS (1886) expanded the ge- nus to include eight species, of which four were new. CALVERT (1903, 1907) described three species (N. aaroni, N. amelia, and N. paya) from southern Texas, Mexico, and Central America. WILLIAMSON (1917), in his seminal revision monographed all known species, described six new species, and included diagnos- tic illustrationsof all known species. It is the only paper which attempted to key all work is valuable for Williamson’s species. Besides the illustrations, the attempt to determine the identity of some of the poorly known Selysian species. Although Williamsonkeyed the males for all species, he did not have material ofN. rufilhorax Selys or N. waltheri Selys. At about the same time, KENNEDY (1917) illustrated the penes of all known species. -
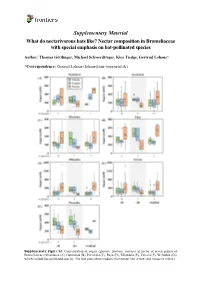
Supplementary Material What Do Nectarivorous Bats Like? Nectar Composition in Bromeliaceae with Special Emphasis on Bat-Pollinated Species
Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S1: Concentration of sugars (glucose, fructose, sucrose) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)) which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S2: Concentration of amino acids (ala, arg, asn, asp, gaba, gln, glu, gly, his, iso, leu, lys, met, phe, pro, ser, thr, trp, tyr, val) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S3: Cation concentrations (Ca2+, K+, Na+, Mg2+) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss.