Date Palm Pollen: Features, Production, Extraction and Pollination Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TAXON:Phoenix Sylvestris SCORE:5.0 RATING:Evaluate
TAXON: Phoenix sylvestris SCORE: 5.0 RATING: Evaluate Taxon: Phoenix sylvestris Family: Arecaceae Common Name(s): date sugar palm Synonym(s): Elate sylvestris L. (basionym) Indian date silver date palm wild date palm Assessor: No Assessor Status: Assessor Approved End Date: 29 Jul 2014 WRA Score: 5.0 Designation: EVALUATE Rating: Evaluate Keywords: Naturalized, Tropical Palm, Spiny, Dioecious, Bird-dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs -

Arizona Landscape Palms
Cooperative Extension ARIZONA LANDSCAPE PALMS ELIZABETH D AVISON Department of Plant Sciences JOHN BEGEMAN Pima County Cooperative Extension AZ1021 • 12/2000 Issued in furtherance of Cooperative Extension work acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona. The University of Arizona College of Agriculture and Life Sciences is an equal opportunity employer authorized to provide research, educational information and other services to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Vietnam Era Veteran's status, or disability. Contents Landscape Use ......................................... 3 Adaptation ................................................ 3 Planting Palms ......................................... 3 Care of Established Palms...................... 5 Diseases and Insect Pests ....................... 6 Palms for Arizona .................................... 6 Feather Palms ........................................... 8 Fan Palms................................................ 12 Palm-like Plants ..................................... 16 This information has been reviewed by university faculty. ag.arizona.edu/pubs/garden/az1121.pdf 2 The luxuriant tropical appearance and stately Adaptation silhouette of palms add much to the Arizona landscape. Palms generally can be grown below the 4000 ft level Few other plants are as striking in low and mid elevation in Arizona. However, microclimate may make the gardens. Although winter frosts and low humidity limit difference between success and failure in a given location. the choices somewhat, a good number of palms are Frost pockets, where nighttime cold air tends to collect, available, ranging from the dwarf Mediterranean Fan should be avoided, especially for the tender species. Palms palm to the massive Canary Island Date palm. -
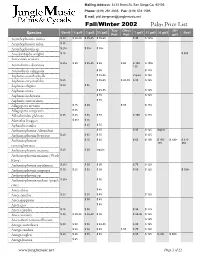
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

The Origin of Monocotyledons from Dicotyledons, Through Self-Adaptation to a Moist
The Origin of Monocotyledons from Dicotyledons, through Self-adaptation to a Moist. or Aquatic Habit.1 BY G. HENSLOW, M.A., F.L.S., F.G.S., F.R.H.S., V.M.H. CONTENTS. SECTIONS. i PAGE 1. INTRODUCTION . 717 2. Evidences from Geology ........... 718 3. Distribution and Percentages of the Natural Orders of Monocotyledons ns com- pared with those of Dicotyledons . 719 4. Degeneracy of Monocotyledons 720 5. Possible Aquatic Origin.of Palms 723 6. Leaves of Large Size characteristic of many Aquatic Plants .... 734 7. Water-storage Organs ........... 724 8. The Requirement of much Water by many Terrestrial Monocotyledons . 724 9. Cycads and Monocotyledons . 725 10. Monocotyledonous Dicotyledons . • 727 11. The Effects of Water upon Roots 731 12. Origin of the formerly called 'Endogenous' Arrangement of the Cauline Bundles of Monocotyledons . ........ 73a 13. The Forms and Structure of Aquatic Leaves are the Result of the Direct Action of Water -735 14. The Reticulated Venation of some Monocotyledons is only imitative of that of Dicotyledons 736 15. Reproductive Organs ............ 737 16. Cytological and Embryological Investigations 738 17. Speculations on the Arrest of one Cotyledon ....... 74° 18. Non-inheritance, Imperfect and Complete Inheritance, of Acquired Characters . 741 19. Isolation and Natural Selection 743 20. CONCLUSION 743 1. INTRODUCTION. EARLY twenty years ago (1892), I pointed out that there is a large N number of coincidences between the morphological and anatomical characters of Monocotyledons and Aquatic Dicotyledons, sufficient, in fact, to justify the conception that the former class had been evolved from the latter. Beyond a limited extent in experiments upon adaptation, the 1 Supplementary Observations and Experiments to ' A Theoretical Origin of Endogens from Exogens, through Self-adaptation to an Aquatic Habit'. -

Hybridization in the Genus Phoenix: a Review
Emir. J. Food Agric. 2013. 25 (11): 831-842 doi: 10.9755/ejfa.v25i11.16660 http://www.ejfa.info/ REVIEW ARTICLE Hybridization in the genus Phoenix: A review Muriel Gros-Balthazard* University of Fribourg, Department of Biology, Biochemistry, Chemin du Musée 10, 1700 Fribourg, Switzerland Abstract The genus Phoenix is composed of 14 species naturally distributed in the Old World. This genus comprises the date palm, Phoenix dactylifera L., cultivated for its fruits, the dates, while other species are grown for food, ornament and religious purposes. Phoenix species were, for these reasons, spread out of their natural distribution area. It is therefore common to find species not naturally sympatric, growing together, in cultivation or in the wild. Phoenix species are interfertile and crossing distinct species leads to fertile hybrid offspring (interspecific hybridization). The introduction of a species in the wild generates gene flows leading to the creation of new hybrids and has conservation implications. In cultivation, such crossings may be spontaneous or are the result of artificial pollination, as several reasons impel doing so. Crossing gives rise to beautiful hybrids and is also useful for the conservation of old palm groves threatened by pests. Moreover, artificial pollination of date palms using another Phoenix species can be of interest given the metaxenic pollen effects. In addition, this process may have some potential benefits in date palm improvements, by the creation of hybrid cultivars. Thus, an increasing need of hybrid detection and characterization exists, particularly as morphology alone is not sufficient for this task. Besides new methods such as traditional and geometric morphometrics that may bring new clues, the advent of genetic and molecular markers helps to detect hybrids, especially based on the combination of nuclear and chloroplastic data. -

Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List
Arizona Department of Water Resources Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Official Regulatory List for the Phoenix Active Management Area Fourth Management Plan Arizona Department of Water Resources 1110 West Washington St. Ste. 310 Phoenix, AZ 85007 www.azwater.gov 602-771-8585 Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Acknowledgements The Phoenix AMA list was prepared in 2004 by the Arizona Department of Water Resources (ADWR) in cooperation with the Landscape Technical Advisory Committee of the Arizona Municipal Water Users Association, comprised of experts from the Desert Botanical Garden, the Arizona Department of Transporation and various municipal, nursery and landscape specialists. ADWR extends its gratitude to the following members of the Plant List Advisory Committee for their generous contribution of time and expertise: Rita Jo Anthony, Wild Seed Judy Mielke, Logan Simpson Design John Augustine, Desert Tree Farm Terry Mikel, U of A Cooperative Extension Robyn Baker, City of Scottsdale Jo Miller, City of Glendale Louisa Ballard, ASU Arboritum Ron Moody, Dixileta Gardens Mike Barry, City of Chandler Ed Mulrean, Arid Zone Trees Richard Bond, City of Tempe Kent Newland, City of Phoenix Donna Difrancesco, City of Mesa Steve Priebe, City of Phornix Joe Ewan, Arizona State University Janet Rademacher, Mountain States Nursery Judy Gausman, AZ Landscape Contractors Assn. Rick Templeton, City of Phoenix Glenn Fahringer, Earth Care Cathy Rymer, Town of Gilbert Cheryl Goar, Arizona Nurssery Assn. Jeff Sargent, City of Peoria Mary Irish, Garden writer Mark Schalliol, ADOT Matt Johnson, U of A Desert Legum Christy Ten Eyck, Ten Eyck Landscape Architects Jeff Lee, City of Mesa Gordon Wahl, ADWR Kirti Mathura, Desert Botanical Garden Karen Young, Town of Gilbert Cover Photo: Blooming Teddy bear cholla (Cylindropuntia bigelovii) at Organ Pipe Cactus National Monutment. -

New Palm Phytoplasma in Florida
1 Texas Phoenix Palm Decline (TPPD) is a palm disease caused by a phytoplasma, a specialized unculturable bacterium without a cell wall. TPPD initially was discovered in the southern coastal area of Texas during 2001 on Canary Island date palm (Phoenix canariensis). TPPD first was identified in the coastal regions of West Central Florida on Phoenix species in 2006. Before the detection of TPPD, the only palm disease in Florida caused by a phytoplasma was lethal yellowing, a systemic palm disease that is transmitted by a common neotropical planthopper (Haplaxius crudus). The symptoms of lethal yellowing are almost identical to TPPD, but the host range and geographical distribution differ. TPPD is closely related to lethal yellowing and the phytoplasma is similar. Information Sources: Harrison, N.A., M. Womack, and M.L. Carpio. 2002. Detection and characterization of a lethal yellowing (16SrIV) group phytoplasma in Canary island date palms affected by lethal decline in Texas. Plant Disease 86(6): 676-681. accessed 12/5/2013 - http://apsjournals.apsnet.org/doi/abs/10.1094/PDIS.2002.86.6.676 Harrison, N. A., E. E. Helmick, M. L. Elliott. 2008. Lethal yellowing-type diseases of palms associated with phytoplasmas newly identified in Florida, USA. Annals of Applied Biology 153:85-94. Harrison, N. A., E. E. Helmick, M. L. Elliott. 2009. First report of a phytoplasma-associated lethal decline of Sabal palmetto in Florida, USA. Plant Pathology 58:792. Harrison, N. and M. Elliott. 2007. Revised 2013. Texas Phoenix Palm Decline. University of Florida, Institute of Food and Agricultural Sciences. Accessed 10-21-13 http://edis.ifas.ufl.edu/pp163 Halbert. -

Phoenix AMA LWUPL
Arizona Department of Water Resources Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Official Regulatory List for the Phoenix Active Management Area Fourth Management Plan Arizona Department of Water Resources 1110 West Washington St. Ste. 310 Phoenix, AZ 85007 www.azwater.gov 602-771-8585 Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Acknowledgements The Phoenix AMA list was prepared in 2004 by the Arizona Department of Water Resources (ADWR) in cooperation with the Landscape Technical Advisory Committee of the Arizona Municipal Water Users Association, comprised of experts from the Desert Botanical Garden, the Arizona Department of Transporation and various municipal, nursery and landscape specialists. ADWR extends its gratitude to the following members of the Plant List Advisory Committee for their generous contribution of time and expertise: Rita Jo Anthony, Wild Seed Judy Mielke, Logan Simpson Design John Augustine, Desert Tree Farm Terry Mikel, U of A Cooperative Extension Robyn Baker, City of Scottsdale Jo Miller, City of Glendale Louisa Ballard, ASU Arboritum Ron Moody, Dixileta Gardens Mike Barry, City of Chandler Ed Mulrean, Arid Zone Trees Richard Bond, City of Tempe Kent Newland, City of Phoenix Donna Difrancesco, City of Mesa Steve Priebe, City of Phornix Joe Ewan, Arizona State University Janet Rademacher, Mountain States Nursery Judy Gausman, AZ Landscape Contractors Assn. Rick Templeton, City of Phoenix Glenn Fahringer, Earth Care Cathy Rymer, Town of Gilbert Cheryl Goar, Arizona Nurssery Assn. Jeff Sargent, City of Peoria Mary Irish, Garden writer Mark Schalliol, ADOT Matt Johnson, U of A Desert Legum Christy Ten Eyck, Ten Eyck Landscape Architects Jeff Lee, City of Mesa Gordon Wahl, ADWR Kirti Mathura, Desert Botanical Garden Karen Young, Town of Gilbert Cover Photo: Blooming Teddy bear cholla (Cylindropuntia bigelovii) at Organ Pipe Cactus National Monutment. -

Phoenix Four River Flora
The Phoenix Four Rivers Flora, Maricopa County, Arizona by Darin Jenke A Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science Approved April 2011 by the Graduate Supervisory Committee: Kathleen B. Pigg, Co-Chair Leslie R. Landrum, Co-Chair Elizabeth Makings ARIZONA STATE UNIVERSITY May 2011 ABSTRACT The Phoenix Four Rivers Flora is an inventory of all the vascular plants growing along the Salt, Gila, New and Agua Fria Rivers, and their tributaries in the Phoenix Metropolitan Area during the years of the study (2009-2011). This floristic inventory documents the plant species and habitats that exist currently in the project area, which has changed dramatically from previous times. The data gathered by the flora project thus not only documents how the current flora has been altered by urbanization, but also will provide a baseline for future ecological studies. The Phoenix Metropolitan Area is a large urbanized region in the Sonoran Desert of Central Arizona, and its rivers are important for the region for many uses including flood control, waste water management, recreation, and gravel mining. The flora of the rivers and tributaries within the project area is extremely diverse; the heterogeneity of the systems being caused by urbanization, stream modification for flood control, gravel mining, and escaped exotic species. Hydrological changes include increased runoff in some areas because of impermeable surfaces (e.g. paved streets) and decreased runoff in other areas due to flood retention basins. The landscaping trade has introduced exotic plant species that have escaped into urban washes and riparian areas. Many of these have established with native species to form novel plant associations. -

The Date Palm in Southern Nevada
THE DATE PALM IN SOUTHERN NEVADA M. L. Robinson, Associate Professor & Area Specialist Brian Brown, Owner China Ranch Date Farm & Grower C. Frank Williams, Professor Emeritus SP-02-12 ORIGIN Phoenix dactylifera is a palm with a long and interesting history. Its origin goes back to ancient times, well before written history. It is a member of the genus Phoenix, which contains about one dozen species of palms. Although other species in this genus produce fruits that are eaten by birds and other animals, Phoenix dactylifera is the only Phoenix species cultivated for its fruit. The date palm is the characteristic vegetation found in the oases of arid areas in the Middle East. It was regarded by the ancient Egyptians as a symbol of fertility, and considered by others as the tree of life. The Greeks and Romans used it as a symbol of triumph, and the Hebrew and Christian cultures as a symbol of peace. Its name, Phoenix, is derived from the Phoenicians who were among the first to describe this plant in their travels. Dactylifera is derived from dactylus for “date” from the Greek dactylos, and fero for “date bearing” or “I bear.” Observations of this plant were first recorded around 5000-6000 BC in Iran, Egypt, and Pakistan. Most likely, these species were wild. The first record of cultivation comes from Lower Mesopotamia around 4000 BC. Later, when the Moors entered Spain, they brought the date with them. Although the Moors were forced out of Spain, the dates stayed and were brought to the Americas by the early Spanish missionaries. -

The Palm Disease and Disorder Issue June 2003
Vol 17. No. 1 The Palm Disease and Disorder Issue June 2003 Landscape Entomology Symposium—WE STILL HAVE ROOM! Due to underwhelming turnout (despite a great program) I have extended the cutoff date for the Landscape Entomology Symposium coming up June 17 at the Saticoy Country Club. We have great speakers on the latest landscape insect problems, 6.5 PCA and 5 arborist CEU hours, great food for a reasonable registration cost of $65.00. If you want to attend the meeting give me a call at 805-645-1458 or mail your registration fee with a check made out to UC REGENTS to Jim Downer, University of California Cooperative Extension, 669 County Square Drive, suite 100, Ventura CA 93003. Palm Disease Note Palms are being increasingly planted in Southern California landscapes as accent and specimen plantings. In many ways, palms are the signature trees of Southern California landscapes and are used to create the Mediterranean, tropical and subtropical “look”. Over the last ten years, dramatic, increased use of Queen palms (Syagrus rommanzoffiana) and both the Canary Island date palm (Phoenix canariensis) and the date palm (Phoenix dactylifera) have flooded landscapes with newly planted palms. Palms, like all plants are susceptible to various biotic and abiotic disease problems. With all the increased planting, we are starting to see more and more problems with palms in the landscape. The intense demand for palms has led to mass production of them in the desert areas of California, along the coast and in the inland valleys. These nursery or field grown plants are then sold and shipped everywhere from Las Vegas, Nevada to San Francisco or used locally near the area of production. -

Palm Maintenance in Historic Districts
PALM PALM DISTRICTS IN HISTORIC MAINTENANCE City of Phoenix Historic Preservation Office 200 W. Washington St., 17th Floor Phoenix, AZ 85003 reservation Planner, Historic Preservation Office Historic Preservation reservation Planner, 1/09 500 P Recreation Department; Design by Erika Finbraaten, Historic Recreation Text by Richard van-C. Adkins, Forestry Supervisor, Parks and Parks by Richard van-C. Adkins, Forestry Supervisor, Text Palms have been a part of the Phoenix area While palms located in their to be patient and wait for the flowering cycle to landscape since the early 1900s. The palm’s natural habitat do not need be complete. Otherwise, if pruned too early, stately appearance has come to be associated not pruning, palms in an urban additional flower stalks may develop and will hang only with the pioneering history of the area, but a environment do require annual from the palm head the remainder of the year. comforting desert oasis feel in the Valley of the maintenance to remove dead Sun. Palms have been planted throughout fronds and fruit stalks that can As a general rule, plan neighborhoods and business districts by residents become unsightly and to begin pruning date and developers, often defining the landscape potentially hazardous. palms in late May and palette of the surrounding community. In the fan palms in mid- city’s historic districts, they are a character June. Cut fronds close defining feature. Severely over- Correct pruning is a key to the petiole base of pruned date palm component to maintaining palm the frond (the petiole health. Many people base is where the indiscriminately cut palms to remove nearly all of Properly pruned fan palm palm frond attaches the fronds.