Hybridization in the Genus Phoenix: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phoenix Sylvestris, Wild Date Palm1 Robert J
FOR 246 Phoenix sylvestris, Wild Date Palm1 Robert J. Northrop, Michael G. Andreu, Melissa H. Friedman, Mary McKenzie, and Heather V. Quintana2 Family Description Arecaceae, palm family. This palm is native to India and southern portions of Pakistan. In both countries, it occurs in areas where there Genus is sparse vegetation mainly composed of scrub species and along flat lands where monsoons occur. Though slow Phoenix is the Latin term for the Greek word that means growing, it can reach heights of up to 50 feet and grows well “date palm.” in areas of the United States where temperatures do not fall below 15°F. Leaves are pinnately compound and blue-green, Species and they can grow to 10 feet in length. Leaflets can reach The species name sylvestris translates from the Latin term approximately 18 inches long and grow opposite to one for “of the forest.” another on the rachis in such a way that the entire leaf looks flat. The petiole, or stem that attaches the leaf to the trunk, is 3 feet long and armed with spines. Young trunks bear Common Names triangular shaped leaf scars (the place where leaves once Wild Date Palm, Toddy Palm, Sugar Date attached to the trunk) that become more diamond-shaped Palm, Silver Date Palm with age. On older trees, aerial roots tend to be present This palm produces edible fruits but it is generally called at the base of the trunk. Yellow inflorescences can reach “wild date palm” to distinguish it from the closely related lengths of 3 feet, are heavily branched, bear small white Phoenix dactylifera, which is known as “date palm” and is blossoms, and grow among the leaves. -

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -

Buy Phoenix Canariensis - 0.5 Kg Seeds Online at Nurserylive | Best Seeds at Lowest Price
Buy phoenix canariensis - 0.5 kg seeds online at nurserylive | Best seeds at lowest price Phoenix canariensis - 0.5 Kg Seeds Phoenix canariensis is hardy palm; spreading deep green leaves in low maintenance. Rating: Not Rated Yet Price Variant price modifier: Base price with tax Price with discount ?999 Salesprice with discount Sales price ?999 Sales price without tax ?999 Discount Tax amount 1 / 3 Buy phoenix canariensis - 0.5 kg seeds online at nurserylive | Best seeds at lowest price Ask a question about this product Description Description for Phoenix Canariensis Phoenix canariensis is the hardy palm and most popular palm species. It has a husk-like stem consisting of wide, dark green leaf bases partly covered with brown, small fibrous hair. The dark green fronds are finely divided and their stalks are a paler green color. The large fronds grow upwards direction from a single crown of palm. Common name(s): Canary Island Date Palm, Pineapple Palm, Canary Date Palm, Slender Date Palm Flower colours: Not defined Bloom time: Not defined Max reacahble height: Up to 10 m Difficulty to grow:: Easy to grow Planting and care Sunlight: Full sunlight Soil: Sandy loam soil Water: Moderately Temperature: 25 to 35 degree C Fertilizer: Use any organic fertilizer Caring for Phoenix Canariensis Watering moderately done. Apply a balanced fertilizer once a month at half strength. Prune or remove old dry fronds. Typical uses of Phoenix Canariensis Special features: Shiny, feathery fronds References 2 / 3 Buy phoenix canariensis - 0.5 kg seeds online at nurserylive | Best seeds at lowest price http://www.plantsrescue.com/phoenix-canariensis/ https://www.houseplantsexpert.com/canary-island-date-palm.html https://www.gardeningknowhow.com/ornamental/trees/canary-palm/canary-island-palm-trees.htm Reviews Monday, 27 July 2020 kamta prasad Friday, 24 July 2020 Rajesh Chandra Friday, 13 March 2020 amitmehta More reviews 3 / 3 Powered by TCPDF (www.tcpdf.org). -

TAXON:Phoenix Sylvestris SCORE:5.0 RATING:Evaluate
TAXON: Phoenix sylvestris SCORE: 5.0 RATING: Evaluate Taxon: Phoenix sylvestris Family: Arecaceae Common Name(s): date sugar palm Synonym(s): Elate sylvestris L. (basionym) Indian date silver date palm wild date palm Assessor: No Assessor Status: Assessor Approved End Date: 29 Jul 2014 WRA Score: 5.0 Designation: EVALUATE Rating: Evaluate Keywords: Naturalized, Tropical Palm, Spiny, Dioecious, Bird-dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs -

Arizona Landscape Palms
Cooperative Extension ARIZONA LANDSCAPE PALMS ELIZABETH D AVISON Department of Plant Sciences JOHN BEGEMAN Pima County Cooperative Extension AZ1021 • 12/2000 Issued in furtherance of Cooperative Extension work acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona. The University of Arizona College of Agriculture and Life Sciences is an equal opportunity employer authorized to provide research, educational information and other services to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Vietnam Era Veteran's status, or disability. Contents Landscape Use ......................................... 3 Adaptation ................................................ 3 Planting Palms ......................................... 3 Care of Established Palms...................... 5 Diseases and Insect Pests ....................... 6 Palms for Arizona .................................... 6 Feather Palms ........................................... 8 Fan Palms................................................ 12 Palm-like Plants ..................................... 16 This information has been reviewed by university faculty. ag.arizona.edu/pubs/garden/az1121.pdf 2 The luxuriant tropical appearance and stately Adaptation silhouette of palms add much to the Arizona landscape. Palms generally can be grown below the 4000 ft level Few other plants are as striking in low and mid elevation in Arizona. However, microclimate may make the gardens. Although winter frosts and low humidity limit difference between success and failure in a given location. the choices somewhat, a good number of palms are Frost pockets, where nighttime cold air tends to collect, available, ranging from the dwarf Mediterranean Fan should be avoided, especially for the tender species. Palms palm to the massive Canary Island Date palm. -
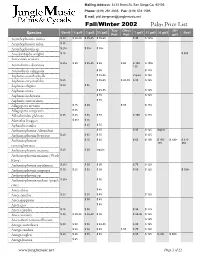
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Landscaper's Guide Working with Palms In
Landscaper’s Guide Landscapers & Working with Nurseries Palms in the Purchasing restricted Palms outside of the quarantine Coachella Valley area and transporting into the protected area is prohibited by law. If you are caught, expect the following actions against you— ♦ Civil Administrative ♦ Criminal ♦ Civil Action Penalties will be imposed on violators. Riverside County PROOF OF PURCHASE MANDATORY Agricultural Commissioner Headquarters & District Office Plant materials found in 4080 Lemon Street, Room 19, Basement P.O. Box 1089, Riverside, CA 95202-1089 violation of the Quarantine Office: (951) 955-3045 law shall be removed at the Facsimile: (951) 955-3012 owner’s expense. Civil fines Indio District Office and Jail time may apply to Office: (760) 863-8291 each violation. Facsimile: (760) 863-7702 California Date Commission P.O. Box 1736 Indio, Ca 92202 Office: (760) 347-4510 Facsimile: (760) 347-6374 Email: [email protected] Website: www.DatesAreGreat.com 04/08 Susceptible Palms Infectious to palm frond; discoloration of leaves on one side of a palm frond and branches. Date Palm Disease the Date Palm, Phoenix dactylifera The disease can be carried on: InteriorInterior QuarantineQuarantine Not Allowable for Transporting and ⇒ Seeds Planting into a Protected Area ⇒ Plants Adopted by the California Department ⇒ Saws Canary Island Date Palm, Phoenix canariensis of Food and Agriculture in 1980, this ⇒ Knives and other tools used quarantine was established to protect “This palm is a known carrier of fusarium wilt for trimming or pruning date fungus infectious to the Senegal Date Palm, P. the California Date industry from palms reclinata and Date Palm P. dactylifera” Fusarium oxysporum, a soil-borne These precautionary measures should be taken Clump Palm (Senegal Date Palm), Phoenix reclinata fungus spread with the Canary Island to prevent the disease from spreading when working on palm trees in the Coachella Valley. -

The Origin of Monocotyledons from Dicotyledons, Through Self-Adaptation to a Moist
The Origin of Monocotyledons from Dicotyledons, through Self-adaptation to a Moist. or Aquatic Habit.1 BY G. HENSLOW, M.A., F.L.S., F.G.S., F.R.H.S., V.M.H. CONTENTS. SECTIONS. i PAGE 1. INTRODUCTION . 717 2. Evidences from Geology ........... 718 3. Distribution and Percentages of the Natural Orders of Monocotyledons ns com- pared with those of Dicotyledons . 719 4. Degeneracy of Monocotyledons 720 5. Possible Aquatic Origin.of Palms 723 6. Leaves of Large Size characteristic of many Aquatic Plants .... 734 7. Water-storage Organs ........... 724 8. The Requirement of much Water by many Terrestrial Monocotyledons . 724 9. Cycads and Monocotyledons . 725 10. Monocotyledonous Dicotyledons . • 727 11. The Effects of Water upon Roots 731 12. Origin of the formerly called 'Endogenous' Arrangement of the Cauline Bundles of Monocotyledons . ........ 73a 13. The Forms and Structure of Aquatic Leaves are the Result of the Direct Action of Water -735 14. The Reticulated Venation of some Monocotyledons is only imitative of that of Dicotyledons 736 15. Reproductive Organs ............ 737 16. Cytological and Embryological Investigations 738 17. Speculations on the Arrest of one Cotyledon ....... 74° 18. Non-inheritance, Imperfect and Complete Inheritance, of Acquired Characters . 741 19. Isolation and Natural Selection 743 20. CONCLUSION 743 1. INTRODUCTION. EARLY twenty years ago (1892), I pointed out that there is a large N number of coincidences between the morphological and anatomical characters of Monocotyledons and Aquatic Dicotyledons, sufficient, in fact, to justify the conception that the former class had been evolved from the latter. Beyond a limited extent in experiments upon adaptation, the 1 Supplementary Observations and Experiments to ' A Theoretical Origin of Endogens from Exogens, through Self-adaptation to an Aquatic Habit'. -

Maquetación 1
Rev. Acad. Canar. Cienc., Vol. XXVII, 357-410 (diciembre de 2015) The botany of the three voyages of Captain James Cook in Macaronesia: an introduction Francisco-Ortega 1,2 *, J., Santos-Guerra 3, A., Romeiras 4,5 , M. M. , Carine 6, M. A. , Sánchez-Pinto 7, L. & Duarte 4,8 *, M. C. 1 International Center for Tropical Botany, Latin American and Caribbean Center Department of Biological Sciences Florida International University, University Park, Miami, Florida, U.S.A. 2 Kushlan Tropical Science Institute, Fairchild Tropical Botanic Garden 10901 Old Cutler Road, Coral Gables, Florida , U.S.A. 3 Calle Guaidil 16, Urbanización Tamarco, Tegueste, Tenerife, Spain 4 Tropical Research Institute (IICT), Faculty of Sciences, University of Lisbon, Campo Grande, Portugal 5 Biosystems and Integrative Sciences Institute (BioISI), Faculty of Sciences University of Lisbon, Campo Grande, Portugal 6 Plants Division, Department of Life Sciences, Natural History Museum Cromwell Road, London, United Kingdom 7 Museo de la Naturaleza y el Hombre, Calle Fuente Morales 2, Santa Cruz de Tenerife, Spain 8 Centre in Biodiversity and Genetic Resources (CIBIO/InBIO), University of Porto Campus Agrário de Vairão, Vairão, Portugal * Corresponding authors: [email protected]; [email protected] ABSTRACT The British naval captain James Cook (1728-1779) was one of the most important figures in the history of scientific exploration. During the 18 th century he was the only ex - plorer to call on the four Macaronesian archipelagos. His first two visits were part of voy - ages that circumnavigated the globe and included celebrated naturalists, notably Sir Joseph Banks (1743-1820) and Daniel Solander (1733-1782) (first voyage) and Johann Reinhold Forster (1729-1798) and his son George Forster (1754-1794) (second voyage). -

Diversidad Genética En Especies Del Género Phoenix L
Universidad Miguel Hernández de Elche (España) Doctorado en Recursos y Tecnologías Agroalimentarias DIVERSIDAD GENÉTICA EN ESPECIES DEL GÉNERO PHOENIX L. TESIS DOCTORAL ENCARNACIÓN CARREÑO SÁNCHEZ ORIHUELA (ESPAÑA) 2017 Diversidad genética en especies del género Phoenix L. Tesis Doctoral realizada por Encarnación Carreño Sánchez, Licenciada en Ciencias Biológicas en la Universidad de Murcia y Máster Universitario en Agroecología, Desarrollo Rural y Agroturismo en la Universidad Miguel Hernández de Elche (Alicante), para la obtención del grado de Doctor. Fdo.: Encarnación Carreño Sánchez Orihuela, 15 de junio de 2017 Dr. José Ramón Díaz Sánchez, Dr. Ingeniero Agrónomo, Catedrático de Universidad y Director del Departamento de Tecnología Agroalimentaria de la Universidad Miguel Hernández, INFORMA: Que atendiendo al informe presentado por los Dres. Concepción Obón de Castro profesora Titular del Departamento de Biología Aplicada de la Universidad Miguel Hernández de Elche, Diego Rivera Núñez Catedrático de Universidad del Departamento de Biología Vegetal de la Universidad de Murcia, y Francisco Alcaraz Ariza Catedrático de Universidad del Departamento de Biología Vegetal de la Universidad de Murcia, la Tesis Doctoral titulada “Diversidad genética en especies del género Phoenix” de la que es autora la licenciada en Biología y Master en Agroecología Desarrollo Rural y Agroturismo Dña. Encarnación Carreño Sánchez ha sido realizada bajo la dirección de los Doctores citados, puede ser presentada para su correspondiente exposición pública. Y para que conste a los efectos oportunos firmo el presente informe en Orihuela a _______de ________ de 2017. Fdo.: Dr. José Ramón Díaz Sánchez Dr. Concepción Obón de Castro, Profesora Titular del Departamento de Biología Aplicada de la Universidad Miguel Hernández de Elche, CERTIFICA: Que la Tesis Doctoral titulada “Diversidad genética en especies del género Phoenix” de la que es autor la licenciada en Biología y Master en Agroecología Desarrollo Rural y Agroturismo Da. -

Washingtonia × Filibusta (Arecaceae: Coryphoideae), a New Hybrid from Cultivation
Hodel, D.R. 2014. Washingtonia × filibusta (Arecaceae: Coryphoideae), a new hybrid from cultivation. Phytoneuron 2014-68: 1–7. Published 1 July 2014. ISSN 2153 733X WASHINGTONIA × FILIBUSTA (ARECACEAE: CORYPHOIDEAE), A NEW HYBRID FROM CULTIVATION DONALD R. HODEL University of California Cooperative Extension 700 W. Main Street Alhambra, California 91801 [email protected] ABSTRACT Washingtonia × filibusta , a hybrid between W. filifera H. Wendl. and W. robusta H. Wendl., is named and described from a cultivated plant in Indio, California. Washingtonia filifera (California fan palm) and W. robusta (Mexican fan palm) have long been cultivated and are especially common landscape subjects in California, western Arizona, and southern Nevada as well as other regions with a suitable climate. They frequently grow side-by-side in landscape settings, providing ample opportunity for hybridization. Hybrid plants are intermediate between the parents and for the past 30 years have become common subjects in the nursery and landscape trade, where they are recognized for their different appearance and cultivation requirements. Washingtonia × filibusta Hort. ex Hodel, sp. hyb. nov. (W. filifera H. Wendl. × W. robusta H. Wendl.). TYPE : CULTIVATED. USA . California. Riverside Co.: Indio, Desert Trace Way, 100 m W of Jackson Street, 1 m elev., 33° 44’ 56.31” N, 116° 13’ 02.23” W, 13 Jun 2014, D.R. Hodel 2040 with K. Greby (holotype: BH). Similar and intermediate to both its parents. Differing from W. filifera in its more slender stem; smaller, denser leaf canopy; smaller, brighter green leaves with a small patch of white tomentum abaxially at petiole and blade junction; and shorter inflorescences. -

Palmae; Coryphoideae) to Non-Native Flora of Tunisia and North Africa
20/1 • 2021, 19–32 DOI: 10.2478/hacq-2020-0015 Further species within Arecaceae (Palmae; Coryphoideae) to non-native flora of Tunisia and North Africa Ridha El Mokni1, 2, 3 Key words: Alien flora, Phoenix, Abstract Washingtonia, Livistona, Monocots. Five new alien taxa are here recorded from Tunisia. Reported taxa (Livistona chinensis, Phoenix canariensis, P. reclinata, P. theophrasti and Washingtonia robusta) Ključne besede: tujerodna flora, belong to the subfamily Coryphoideae (Arecaceae). Updated nomenclature, brief Phoenix, Washingtonia, Livistona, descriptions, general and national distributions are provided for each species. enokaličnice. Livistona chinensis and Phoenix theophrasti are here reported for the first time in North Africa. Identification keys are also provided. Izvleček V članku predstavljamo pet novih tujerodnih taksonov iz Tunizije. Vsi zabeleženi taksoni (Livistona chinensis, Phoenix canariensis, P. reclinata, P. theophrasti and Washingtonia robusta) pripadajo poddružini Coryphoideae (Arecaceae). Za vsako vrsto smo prikazali posodobljeno nomenklaturo, kratek opis, splošno in nacionalno razširjenost. Livistona chinensis in Phoenix theophrasti sta prvič zabeleženi v severni Afriki. Predstavili smo tudi določevalne ključe. Received: 24. 4. 2020 Revision received: 8. 6. 2020 Accepted: 10. 7. 2020 1 Department of Pharmaceutical Sciences “A”, Laboratory of Botany, Cryptogamy and plant Biology, Faculty of Pharmacy of Monastir, Avenue Avicenna, 5000-Monastir, University of Monastir, Tunisia. E-mail: [email protected] 2 Department of Silvo-pastoral Resources, Laboratory of Silvo-pastoral Resources, Silvo-Pastoral Institute of Tabarka, BP. 345, 8110-Tabarka, University of Jendouba, Tunisia. 3 University of Carthage, Laboratory of Forest Ecology, National Research Institute of Rural Engineering, Water and Forests, Ariana 2080, Tunisia. 19 Ridha El Mokni 20/1 • 2021, 19–32 Further species within Arecaceae (Palmae; Coryphoideae) to non-native flora of Tunisia and North Africa cords.