Bovine Tuberculosis | Monograph 15 • • •
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Natural Gas: Interim Fuel & 25 the First Nuclear Power Plant Bangladesh Perspective of the World
Editor Fortnightly Magazine, Vol 18, Issue 1, June 16-30 Mollah M Amzad Hossain Advisory Editor Anwarul Islam Tarek Mortuza Ahmad Faruque Saiful Amin International Editor Dr. Nafis Ahmed Contributing Editors Saleque Sufi Online Editor GSM Shamsuzzoha (Nasim) Managing Editor Afroza Hossain The national budget for FY2020-21 placed by Finance Minister AHM Mustafa Magazine Administrator Kamal in parliament on June 11 was one of the most challenging tasks of AKM Shamsul Hoque economic and financial management of the state since its independence. Kamal Reporters Arunima Hossain had to deal with this tough job amid the Covid-19 pandemic that has slowed Jannatul Ferdushy Sova down the economy. It was not surprising that he would be cautious in spending Assistant Online Editor in the next fiscal year since the economy is under severe pressure. In the energy Aditya Hossain Design & Graphics and power sector he proposed a cut in budgetary allocation by 4.6 percent. Md. Monirul Islam However, experts in the sector expressed mixed reaction to his plan for energy Photography sector. Apparently there was no change in the budgetary measures for the Bulbul Ahmed government’s existing plans though it was suggested by the experts ahead of the Production Mufazzal Hossain Joy budget announcement. The experts earlier suggested that the government Computer Graphics should go slow with the development projects in the sector which were not in Md. Uzzal Hossain the process of implementation. Instead, they recommended, the funds should be Circulation Assistant directed to sectors that deserve emergency response due to the adverse impact Khokan Chandra Das of the pandemic. -

Annex 13 Master Plan on Sswrd in Mymensingh District
ANNEX 13 MASTER PLAN ON SSWRD IN MYMENSINGH DISTRICT JAPAN INTERNATIONAL COOPERATION AGENCY (JICA) MINISTRY OF LOCAL GOVERNMENT, RURAL DEVELOPMENT AND COOPERATIVES (MLGRD&C) LOCAL GOVERNMENT ENGINEERING DEPARTMENT (LGED) MASTER PLAN STUDY ON SMALL SCALE WATER RESOURCES DEVELOPMENT FOR POVERTY ALLEVIATION THROUGH EFFECTIVE USE OF SURFACE WATER IN GREATER MYMENSINGH MASTER PLAN ON SMALL SCALE WATER RESOURCES DEVELOPMENT IN MYMENSINGH DISTRICT NOVEMBER 2005 PACIFIC CONSULTANTS INTERNATIONAL (PCI), JAPAN JICA MASTER PLAN STUDY ON SMALL SCALE WATER RESOURCES DEVELOPMENT FOR POVERTY ALLEVIATION THROUGH EFFECTIVE USE OF SURFACE WATER IN GREATER MYMENSINGH MASTER PLAN ON SMALL SCALE WATER RESOURCES DEVELOPMENT IN MYMENSINGH DISTRICT Map of Mymensingh District Chapter 1 Outline of the Master Plan Study 1.1 Background ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 1 1.2 Objectives and Scope of the Study ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 1 1.3 The Study Area ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 2 1.4 Counterparts of the Study ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 2 1.5 Survey and Workshops conducted in the Study ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 3 Chapter 2 Mymensingh District 2.1 General Conditions ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 4 2.2 Natural Conditions ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 4 2.3 Socio-economic Conditions ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 5 2.4 Agriculture in the District ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 5 2.5 Fisheries -

Census Data/Projections, 1999 & 2000
Census data/Projections, 1999 & 2000 Jan - June and July - December Relief Bens., Supplementary Feeding Bens. TIGRAY Zone 1994 census 1999 beneficiaries - May '99 2000 beneficiaries - Jan 2000 July - Dec 2000 Bens Zone ID/prior Wereda Total Pop. 1999 Pop. 1999 Bens. 1999 bens % 2000 Pop. 2000 Bens 2000 bens Bens. Sup. July - Dec Bens July - Dec Bens ity Estimate of Pop. Estimate % of Pop. Feeding % of pop 1 ASEGEDE TSIMBELA 96,115 111,424 114,766 1 KAFTA HUMERA 48,690 56,445 58,138 1 LAELAY ADIYABO 79,832 92,547 5,590 6% 95,324 7,800 8% 11,300 12% Western 1 MEDEBAY ZANA 97,237 112,724 116,106 2,100 2% 4,180 4% 1 TAHTAY ADIYABO 80,934 93,825 6,420 7% 96,639 18,300 19% 24,047 25% 1 TAHTAY KORARO 83,492 96,790 99,694 2,800 3% 2,800 3% 1 TSEGEDE 59,846 69,378 71,459 1 TSILEMTI 97,630 113,180 37,990 34% 116,575 43,000 37% 15,050 46,074 40% 1 WELKAIT 90,186 104,550 107,687 Sub Total 733,962 850,863 50,000 6% 876,389 74,000 8% 15,050 88,401 10% *2 ABERGELE 58,373 67,670 11,480 17% 69,700 52,200 75% 18,270 67,430 97% *2 ADWA 109,203 126,596 9,940 8% 130,394 39,600 30% 13,860 58,600 45% 2 DEGUA TEMBEN 89,037 103,218 7,360 7% 106,315 34,000 32% 11,900 44,000 41% Central 2 ENTICHO 131,168 152,060 22,850 15% 156,621 82,300 53% 28,805 92,300 59% 2 KOLA TEMBEN 113,712 131,823 12,040 9% 135,778 62,700 46% 21,945 67,700 50% 2 LAELAY MAYCHEW 90,123 104,477 3,840 4% 107,612 19,600 18% 6,860 22,941 21% 2 MEREB LEHE 78,094 90,532 14,900 16% 93,248 57,500 62% 20,125 75,158 81% *2 NAEDER ADET 84,942 98,471 15,000 15% 101,425 40,800 40% 14,280 62,803 62% 2 -
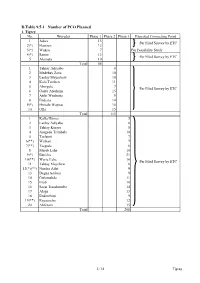
D.Table 9.5-1 Number of PCO Planned 1
D.Table 9.5-1 Number of PCO Planned 1. Tigrey No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Adwa 13 Per Filed Survey by ETC 2(*) Hawzen 12 3(*) Wukro 7 Per Feasibility Study 4(*) Samre 13 Per Filed Survey by ETC 5 Alamata 10 Total 55 1 Tahtay Adiyabo 8 2 Medebay Zana 10 3 Laelay Mayechew 10 4 Kola Temben 11 5 Abergele 7 Per Filed Survey by ETC 6 Ganta Afeshum 15 7 Atsbi Wenberta 9 8 Enderta 14 9(*) Hintalo Wajirat 16 10 Ofla 15 Total 115 1 Kafta Humer 5 2 Laelay Adiyabo 8 3 Tahtay Koraro 8 4 Asegede Tsimbela 10 5 Tselemti 7 6(**) Welkait 7 7(**) Tsegede 6 8 Mereb Lehe 10 9(*) Enticho 21 10(**) Werie Lehe 16 Per Filed Survey by ETC 11 Tahtay Maychew 8 12(*)(**) Naeder Adet 9 13 Degua temben 9 14 Gulomahda 11 15 Erob 10 16 Saesi Tsaedaemba 14 17 Alage 13 18 Endmehoni 9 19(**) Rayaazebo 12 20 Ahferom 15 Total 208 1/14 Tigrey D.Table 9.5-1 Number of PCO Planned 2. Affar No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Ayisaita 3 2 Dubti 5 Per Filed Survey by ETC 3 Chifra 2 Total 10 1(*) Mile 1 2(*) Elidar 1 3 Koneba 4 4 Berahle 4 Per Filed Survey by ETC 5 Amibara 5 6 Gewane 1 7 Ewa 1 8 Dewele 1 Total 18 1 Ere Bti 1 2 Abala 2 3 Megale 1 4 Dalul 4 5 Afdera 1 6 Awash Fentale 3 7 Dulecha 1 8 Bure Mudaytu 1 Per Filed Survey by ETC 9 Arboba Special Woreda 1 10 Aura 1 11 Teru 1 12 Yalo 1 13 Gulina 1 14 Telalak 1 15 Simurobi 1 Total 21 2/14 Affar D.Table 9.5-1 Number of PCO Planned 3. -
Štýl Nadpis 1
119 ETHNOLOGIA ACTUALIS Vol. 20, No. 1/2020 Yohannes TESFAYE GETACHEW A History of Koshe Town in South-Central Ethiopia from 1941 to 1991 A History of Koshe Town in South-Central Ethiopia from 1941 to 1991 YOHANNES TESFAYE GETACHEW Koshe General Secondary and Preparatory School, Koshe Town, Ethiopia [email protected] ABSTRACT Koshe town is the administrative and commercial center of Mareko woreda.1 It is found in Gurage Zone Southern Nation Nationalities and Peoples Regional State. According to the tradition the origin of the name “Koshe” is originated from the plant which called by the name Koshe which abundantly grow in the area. The establishment of Koshe town is directly associated with the five years Italian occupation. Due to the expansion of patriotic movement in the area Italian officials of the area forced to establish additional camp in the area in a particular place Koshe. This paper explores the role of Fascist Italy for the establishment of Koshe town. The former weekly market shifted its location and established around the Italian camp. Following the evacuation of Fascist Italy the Ethiopian governments control the area. During the government of Emperor Haile Selassie Koshe town got some important developmental programs. The most important development was the opening of the first school by the effort of the Swedes.2 The Military regime (Derg)3 also provided important inputs for the 1 It is an administrative unit in Ethiopia, literally means district. 2 Between 1924 and 1952 the Swedes particularly SEM (the Swedish Evangelical Mission) was active in Ethiopia to assist the country in many sects particularly they opened clinics and schools in all twelve provinces (VIVECA 1977:186). -

Annual Report 2018
ANNUAL REPORT 2018 Sabalamby Unnayan Samity (SUS) ANNUAL REPORT 2 0 1 8 (January-December) Chief Adviser Begum Rokeya ANNUAL Editorial Adviser Kazi Sohul Ahmed Showpoun Kumar Paul REPORT 2018 Narayan Chandra Sarker Editor Krishibid Altafur Rahman Selim Editorial Associate Golam Mostafa Rezu Murshed Iqbal Abdur Razzak Khaled Ehtesham Published in March 2019 Sabalamby Unnayan Samity (SUS) Shibgonj Road, Netrakona Email: [email protected] www.sabalamby.org Annual Report 2018 I I am very much glad to present the officials, representatives of network orga- Annual Report 2018 of Sabalamby nizations and development partners for Unnayan Samity (SUS). SUS is imple- their sincere cooperation. I offer my menting different development friendly heartfelt gratitude to the staffs of SUS for programs for improving the livelihood their hard work. status of the poor and disadvantaged section of the society. SUS works to eliminate discrimination and exploitation and help people for their This annual report is the reflection of equal opportunity and dignity. May SUS’s works of 2018. SUS is going development effort of SUS continue forward through its steadiness, compe- towards establishing a prosperous coun- tency, innovation and diversification. try. We are indebted to the stakeholder of different categories who provided their necessary support and valuable informa- tion to keep the initiatives on track. I pay my thanks and gratitude to the Roushan Akhtar Message from members of SUS general and executive Chairman the Chairman committee, different government SUS Executive Committee Annual Report 2018 II I have the pleasure to present our Annual SUS has faced many challenges in Report 2018 to the development partners, continuing its development efforts. -

Socio-Economic, Environmental, and Behavioural Factors Associated with the Occurrence of Diarrhoeal Disease Among Under-Five
SOCIO-ECONOMIC, ENVIRONMENTAL, AND BEHAVIOURAL FACTORS ASSOCIATED WITH THE OCCURRENCE OF DIARRHOEAL DISEASE AMONG UNDER-FIVE CHILDREN, MESKANENA MAREKO WOREDA, SOUTHERN ETHIOPIA. BY TEKLU MULUGETA (B.Sc.) A THESIS SUBMITTED TO THE SCHOOL OF GRADUATE STUDIES OF ADDIS ABABA UNIVERSITY IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTERS OF PUBLIC HEALTH IN THE DEPARTMENT OF COMMUNITY HEALTH MAY 2003 ADDIS ABABA DEDICATION This work is dedicated to my beloved wife Tigist Bogale whose encouragement and help gave me strength to successfully finish this MPH course. I would like also to dedicate this thesis to our son Mussie Teklu who is only two, and also to all under-five children in the study area. ACKNOWLEDGEMENTS The financial and material support for the study was obtained from Addis Ababa University, Medical Faculty, Community Health Department. I am very grateful to my advisors Drs. Abera Kumie and Nigussie Deyessa. The advice, encouragement and guidance of my advisors was so helpful to this study. Special thanks also go to Professor Yemane Berhane for his valuable comments from the initial stage of this project. The unlimited assistance of Sr. Mitike Molla, Research assistant, Butajira Rural Health Program, and W/ro. Shewbeza Yusuf, field coordinator, is very much appreciated. The enumerators and supervisors of the Butajira Rural Health Program also deserve special thanks. The hospitality and cooperation of the population of the study site in general, and the study subjects in particular are highly appreciated. My heartfelt thanks also go to Ato Wondwossen Bekele for his unreserved assistance in the process of data collection and analysis. -

Department of Sociology University of Dhaka Dhaka University Institutional Repository
THE NATURE AND EXTENT OF HOMICIDE IN BANGLADESH: A CONTENT ANALYSIS ON REPORTS OF MURDER IN DAILY NEWSPAPERS T. M. Abdullah-Al-Fuad June 2016 Department of Sociology University of Dhaka Dhaka University Institutional Repository THE NATURE AND EXTENT OF HOMICIDE IN BANGLADESH: A CONTENT ANALYSIS ON REPORTS OF MURDER IN DAILY NEWSPAPERS T. M. Abdullah-Al-Fuad Reg no. 111 Session: 2011-2012 Submitted in partial fulfillment of the requirements of the degree of Master of Philosophy June 2016 Department of Sociology University of Dhaka Dhaka University Institutional Repository DEDICATION To my parents and sister Dhaka University Institutional Repository Abstract As homicide is one of the most comparable and accurate indicators for measuring violence, the aim of this study is to improve understanding of criminal violence by providing a wealth of information about where homicide occurs and what is the current nature and trend, what are the socio-demographic characteristics of homicide offender and its victim, about who is most at risk, why they are at risk, what are the relationship between victim and offender and exactly how their lives are taken from them. Additionally, homicide patterns over time shed light on regional differences, especially when looking at long-term trends. The connection between violence, security and development, within the broader context of the rule of law, is an important factor to be considered. Since its impact goes beyond the loss of human life and can create a climate of fear and uncertainty, intentional homicide (and violent crime) is a threat to the population. Homicide data can therefore play an important role in monitoring security and justice. -

Zila Parishad
Zila Parishad Mymensingh www.zpmymensingh.org.bd Memo No- 46.42.6100.002.07.001.20-1274 Date: 08 November, 2020 Invitation for Tender (works) e-Tender Notice No-06/2020-2021 (NCT, LTM) This e-Tender is invited in the National e-GP System Portal (www.eprocure.gov.bd) for the procurement of following works: SL e-Tender Tender Publication Tender Closing & Package & Name of work No. ID No. (Date & Time) Opening (Date & Time) 49/eGP/ADP/2019-20 (1) Distribution of fans in various educational and religious institutions under Dhobaura upazila (2) (A) Dr. Alman of Dhobaura village (b) Rahima Khatun of Kashinathpur village (c) Raichul Islam Akash of Ghilagarh village (d) Mosharraf Hossain of Langaljora village (e) Abdur Rouf of Langaljora village (f) Kali Mia of Kharia village At the junction (h) 10-Nov-2020 25-Nov-2020 01. 511913 Rafiqul Islam of Krishtapur village (i) Babul Akand of Kharia village (j) Abdur Rashid Member of Ghagutiarpar village (k) 10:00:00 15:00:00 Siddique Member of Tangari village (l) Chan Miah of Gobindpur village (m) Chhakina Khatun of Gobindpur village (n) Serida Khatun of Chanatia village and (o) Russia Khatun of Raghurampur village A total of 15 tubewells were installed on the side of the road in front of their houses under Dhobaura Upazila during the during the FY/2019-2020. 79/e-GP/Rev/2019-20 (1) Development of Barera Loren Mountain Academy (2) Development of Bhatighagra Baba Nazir Uddin Shah Mazar (3) Development of Bir Muktijoddha Principal Matiur Rahman 10-Nov-2020 25-Nov-2020 02. -

Fig.4. the Prevalence of Infertility in Butajira, Ethiopia, 1999
SCHOOL OF GRADUATE STUDIES - INFERTILITY IN RURAL ETHIOPIA THESIS PRESENTED TO THE SCHOOL OF GRADUATE STUDIES ADDIS AREBA UNIVERSITY IN PARTIAL FUIFll-MENT OF THE REQUIREMENT FOR THE DEGREE OF MASTERS OF PUBLIC HEALTH Ashenqfi Haile Haikmarimn, MD December, 1999. ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES Infertility in Rural Ethiopia By Ashenafi Haile, MD Department of Community Health Faculty of Medicine, Addis Ababa University ,,' Approved by the Examining Board Dr. Yemane Berhane Chairman, Department Graduate Committee Dr. Yemane Berhane Advisor Prof. Mohsen Gadallah Examiner _ Dr. Misganaw Fantahun Examiner DEDICATION To my brothe r Dr. Mitiku Haile, his wife Hiwot Abraham, my wife Alem Genanaw and my daughter Higewengel Ashenafi. TABLE OF CONTENTS Title Page List of tables i List of figures ii Acknowledgments iii 1. Abstract . iv 2. Introduction 1 3. Review of the literature 3 4. Objectives of the study 11 5. Material s and methods 12 5.1 The study design 12 5.2 The study area and population 12 5 .3 Sample size . 17 5.4 Study variables 18 5.5 Data collection 20 5.6 Data analysis . 22 5.7 Operational defi nition 22 6 . Resul ts 24 7. Di scussion 45 8. Conclusion 49 9. Recommendations 50 10. References 50 11. Appendix Questionnaire in English . 54 List of tables Page Table 1. The socio-demographic characterstics of the study population . 25 Table 2. Fertility status by selected socio-demographic characterstics of the women in Butajira, Ethiopia,1999. 29 Table 3. The relationship between selected reproductive characteristics of women and secondary infertility in Butajira, Ethiopia, 1999. -

Irrigation and Water for Sustainable Development: Proceedings of The
2nd Forum on Irrigation & Water for Sustainable Development 15 –16 December, 2008 Ghion Hotel, Addis Ababa, Ethiopia Photo credit: Apollo Habtamu Compiled by: Seleshi B. Awulachew, Teklu Erkossa and Yodit Balcha Organized by: Ethiopia National Irrigation Steering Committee Sponsored by: International Water Management Institute (IWMI) Ministry of Agriculture and Rural Development (MoARD) Ministry of Water Resources (MoWR) United States Aid and International Development (USAID) Japan International Cooperation Agency (JICA) Table of Contents ACRONYMS AND ABBREVIATIONS.............................................................................................................. II ACKNOWLEDGEMENTS .............................................................................................................................. III WELCOMING ADDRESS ............................................................................................................................... V OPENING ADDRESS .................................................................................................................................... IX POLICY, STRATEGIES AND INVESTMENTS...................................................................................... 1 TAKING FORWARD THE GROWTH AGENDA OF THE PASDEP: FROM CONCEPT TO ACTION 2 THE ROLE OF THE ETHIOPIAN STRATEGIC INVESTMENT FRAMEWORK FOR SUSTAINABLE LAND MANAGEMENT (ESIF-SLM) IN IRRIGATION DEVELOPMENT............... 2 SMALL-SCALE IRRIGATION DEVELOPMENT INTERVENTIONS UNDER IFAD-SUPPORTED PROJECTS ...............................................................................................................................................