Evaluation and Management of the Patient with a Neck Mass • Introduction • Anatomical Consideration • Diagnostic Steps • DDX • Some Examples • Summary Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseases of Salivary Glands: Review
ISSN: 1812–1217 Diseases of Salivary Glands: Review Alhan D Al-Moula Department of Dental Basic Science BDS, MSc (Assist Lect) College of Dentistry, University of Mosul اخلﻻضة امخجوًف امفموي تُئة رطبة، حتخوي ػىل طبلة ركِلة من امسائل ثدغى انوؼاب ثغطي امسطوح ادلاخوَة و متﻷ امفراغات تني ااطَة امفموًة و اﻷس نان. انوؼاب سائل مؼلد، ًنذج من امغدد انوؼاتَة، اذلي ًوؼة دورا" ىاما" يف اﶈافظة ػىل سﻻمة امفم. املرىض اذلٍن ؼًاهون من هلص يف اﻷفراز انوؼايب حكون دلهيم مشبلك يف اﻷلك، امخحدث، و امبوع و ًطبحون غرضة مﻷههتاابت يف اﻷغش َة ااطَة و امنخر املندرش يف اﻷس نان. ًوخد ثﻻثة أزواج من امغدد انوؼاتَة ام ئرُسة – امغدة امنكفِة، امغدة حتت امفكِة، و حتت انوساهَة، موضؼيا ٍكون خارج امخجوًف امفموي، يف حمفظة و ميخد هظاهما املنَوي مَفرغ افرازاهتا. وًوخد أًضا" امؼدًد من امغدد انوؼاتَة امطغرية ، انوساهَة، اتحنكِة، ادلىوزيًة، انوساهَة احلنكِة وما كبل امرخوًة، ٍكون موضؼيا مﻷسفل و مضن امغشاء ااطي، غري حماطة مبحفظة مع هجاز كنَوي كطري. افرازات امغدد انوؼاتَة ام ئرُسة مُست مدشاهبة. امغدة امفكِة ثفرز مؼاب مطيل غين ابﻷمِﻻز، وامغدة حتت امفكِة ثنذج مؼاب غين اباط، أما امغدة حتت انوساهَة ثنذج مؼااب" مزخا". ثبؼا" ميذه اﻷخذﻻفات، انوؼاب املوحود يق امفم ٌشار امَو مكزجي. ح كرَة املزجي انوؼايب مُس ثس َطا" واملادة اﻷضافِة اموػة من لك املفرزات انوؼاتَة، اكمؼدًد من امربوثُنات ثنذلل ثرسػة وثوخطق هبدروكس َل اﻷتُذاًت مﻷس نان و سطوح ااطَة امفموًة. ثبدأ أمراض امغدد انوؼاتَة ػادة تخغريات اندرة يف املفرزات و ام كرتَة، وىذه امخغريات ثؤثر اثهواي" من خﻻل جشلك انووحية اجلرثومِة و املوح، اميت تدورىا ثؤدي اىل خنور مذفش َة وأمراض وس َج دامعة. ىذه اﻷمراض ميكن أن ثطبح شدًدة تؼد املؼاجلة امشؼاغَة ﻷن امؼدًد من احلاﻻت اجليازًة )مثل امسكري، امخوَف اهكُيس( ثؤثر يف اجلراين انوؼايب، و ٌش خيك املرض من حفاف يف امفم. -

Gross Anatomy
www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY www.BookOfLinks.com Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the authors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the infor- mation contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY David A. Morton, PhD Associate Professor Anatomy Director Department of Neurobiology and Anatomy University of Utah School of Medicine Salt Lake City, Utah K. Bo Foreman, PhD, PT Assistant Professor Anatomy Director University of Utah College of Health Salt Lake City, Utah Kurt H. -

432 Surgery Team Leaders
3 Common Neck Swellings Done By: Reviewed By: Othman.T.AlMutairi Ghadah Alharbi COLOR GUIDE: • Females' Notes • Males' Notes • Important • Additional Outlines Common Anatomy of the Neck Neck Ranula Swellings Dermoid cyst Thyroglossal cyst Branchial cysts Laryngocele Carotid body tumor Hemangioma Cystic Hygroma Inflammatory lymphadenopathy Malignant lymphadenopathy Thyroid related abnormalities Submandibular gland related abnormalities Sjogren's syndrome 1 Anatomy of the Neck: Quadrangular area (1): A quadrangular area can be delineated on the side of the neck. This area is subdivided by an obliquely prominent sternocleidomastoid muscle into anterior and posterior cervical triangles. Anterior cervical triangle is subdivided into four smaller triangles: -Submandibular triangle: Contains the submandibular salivary gland, hypoglossal nerve, mylohyiod muscle, and facial nerve. -Carotid triangle: Contains the carotid arteries and branches, internal jugular vein, and vagus nerve. -Omotracheal triangle: Includes the infrahyoid musculature and thyroid glands with the parathyroid glands. -Submental triangle: Beneath the chin. Figure 1: Anterior cervical muscles. 2 Posterior cervical triangle: The inferior belly of the omohyoid divides it into two triangles: -Occipital triangle: The contents include the accessory nerve, supraclavicular nerves, and upper brachial plexus. -Subclavian triangle: The contents include the supraclavicular nerves, Subclavian vessels, brachial plexus, suprascapular vessels, transverse cervical vessels, external jugular vein, and the nerve to the Subclavian muscle. The main arteries in the neck are the common carotids arising differently, one on each side. On the right, the common carotid arises at the bifurcation of the brachiocephalic trunk behind the sternoclavicular joint; on the left, it arises from the highest point on arch of the aorta in the chest. -

A Guide to Salivary Gland Disorders the Salivary Glands May Be Affected by a Wide Range of Neoplastic and Inflammatory
MedicineToday PEER REVIEWED ARTICLE CPD 1 POINT A guide to salivary gland disorders The salivary glands may be affected by a wide range of neoplastic and inflammatory disorders. This article reviews the common salivary gland disorders encountered in general practice. RON BOVA The salivary glands include the parotid glands, examination are often adequate to recognise and MB BS, MS, FRACS submandibular glands and sublingual glands differentiate many of these conditions. A wide (Figure 1). There are also hundreds of minor sali- array of benign and malignant neoplasms may also Dr Bova is an ENT, Head and vary glands located in the mucosa of the hard and affect the salivary glands and a neoplasia should Neck Surgeon, St Vincent’s soft palate, oral cavity, lips, tongue and oro - always be considered when assessing a salivary Hospital, Sydney, NSW. pharynx. The parotid gland lies in the preauricular gland mass. region and extends inferiorly over the angle of the mandible. The parotid duct courses anteriorly Inflammatory disorders from the parotid gland and enters the mouth Acute sialadenitis through the buccal mucosa adjacent to the second Acute inflammation of the salivary glands is usu- upper molar tooth. The submandibular gland lies ally of viral or bacterial origin. Mumps is the most in the submandibular triangle and its duct passes common causative viral illness, typically affecting anteriorly along the floor of the mouth to enter the parotid glands bilaterally. Children are most adjacent to the frenulum of the tongue. The sub- often affected, with peak incidence occurring at lingual glands are small glands that lie just beneath approximately 4 to 6 years of age. -

Surface Anatomy
BODY ORIENTATION OUTLINE 13.1 A Regional Approach to Surface Anatomy 398 13.2 Head Region 398 13.2a Cranium 399 13 13.2b Face 399 13.3 Neck Region 399 13.4 Trunk Region 401 13.4a Thorax 401 Surface 13.4b Abdominopelvic Region 403 13.4c Back 404 13.5 Shoulder and Upper Limb Region 405 13.5a Shoulder 405 Anatomy 13.5b Axilla 405 13.5c Arm 405 13.5d Forearm 406 13.5e Hand 406 13.6 Lower Limb Region 408 13.6a Gluteal Region 408 13.6b Thigh 408 13.6c Leg 409 13.6d Foot 411 MODULE 1: BODY ORIENTATION mck78097_ch13_397-414.indd 397 2/14/11 3:28 PM 398 Chapter Thirteen Surface Anatomy magine this scenario: An unconscious patient has been brought Health-care professionals rely on four techniques when I to the emergency room. Although the patient cannot tell the ER examining surface anatomy. Using visual inspection, they directly physician what is wrong or “where it hurts,” the doctor can assess observe the structure and markings of surface features. Through some of the injuries by observing surface anatomy, including: palpation (pal-pā sh ́ ŭ n) (feeling with firm pressure or perceiving by the sense of touch), they precisely locate and identify anatomic ■ Locating pulse points to determine the patient’s heart rate and features under the skin. Using percussion (per-kush ̆ ́ŭn), they tap pulse strength firmly on specific body sites to detect resonating vibrations. And ■ Palpating the bones under the skin to determine if a via auscultation (aws-ku ̆l-tā sh ́ un), ̆ they listen to sounds emitted fracture has occurred from organs. -
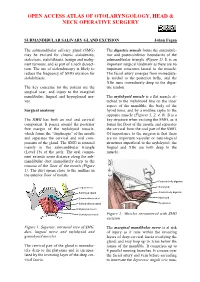
Submandibular Gland Excision
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY SUBMANDIBULAR SALIVARY GLAND EXCISION Johan Fagan The submandibular salivary gland (SMG) The digastric muscle forms the anteroinfe- may be excised for chronic sialadenitis, rior and posteroinferior boundaries of the sialectasis, sialolithiasis, benign and malig- submandibular triangle (Figure 2). It is an nant tumours, and as part of a neck dissect- important surgical landmark as there are no tion. The use of sialendoscopy is likely to important structures lateral to the muscle. reduce the frequency of SMG excision for The facial artery emerges from immediate- sialolithiasis. ly medial to the posterior belly, and the XIIn runs immediately deep to the digas- The key concerns for the patient are the tric tendon. surgical scar, and injury to the marginal mandibular, lingual and hypoglossal ner- The mylohyoid muscle is a flat muscle at- ves. tached to the mylohyoid line on the inner aspect of the mandible, the body of the Surgical anatomy hyoid bone, and by a midline raphe to the opposite muscle (Figures 1, 2, 4, 8). It is a The SMG has both an oral and cervical key structure when excising the SMG, as it component. It passes around the posterior forms the floor of the mouth, and separates free margin of the mylohyoid muscle, the cervical from the oral part of the SMG. which forms the “diaphragm” of the mouth Of importance to the surgeon is that there and separates the cervical and oral com- are no important vascular or neurological ponents of the gland. The SMG is situated structures superficial to the mylohyoid; the mainly in the submandibular triangle lingual and XIIn are both deep to the (Level 1b) of the neck. -

Anatomy Module 3. Muscles. Materials for Colloquium Preparation
Section 3. Muscles 1 Trapezius muscle functions (m. trapezius): brings the scapula to the vertebral column when the scapulae are stable extends the neck, which is the motion of bending the neck straight back work as auxiliary respiratory muscles extends lumbar spine when unilateral contraction - slightly rotates face in the opposite direction 2 Functions of the latissimus dorsi muscle (m. latissimus dorsi): flexes the shoulder extends the shoulder rotates the shoulder inwards (internal rotation) adducts the arm to the body pulls up the body to the arms 3 Levator scapula functions (m. levator scapulae): takes part in breathing when the spine is fixed, levator scapulae elevates the scapula and rotates its inferior angle medially when the shoulder is fixed, levator scapula flexes to the same side the cervical spine rotates the arm inwards rotates the arm outward 4 Minor and major rhomboid muscles function: (mm. rhomboidei major et minor) take part in breathing retract the scapula, pulling it towards the vertebral column, while moving it upward bend the head to the same side as the acting muscle tilt the head in the opposite direction adducts the arm 5 Serratus posterior superior muscle function (m. serratus posterior superior): brings the ribs closer to the scapula lift the arm depresses the arm tilts the spine column to its' side elevates ribs 6 Serratus posterior inferior muscle function (m. serratus posterior inferior): elevates the ribs depresses the ribs lift the shoulder depresses the shoulder tilts the spine column to its' side 7 Latissimus dorsi muscle functions (m. latissimus dorsi): depresses lifted arm takes part in breathing (auxiliary respiratory muscle) flexes the shoulder rotates the arm outward rotates the arm inwards 8 Sources of muscle development are: sclerotome dermatome truncal myotomes gill arches mesenchyme cephalic myotomes 9 Muscle work can be: addacting overcoming ceding restraining deflecting 10 Intrinsic back muscles (autochthonous) are: minor and major rhomboid muscles (mm. -

Hemiageusia, Hemianaesthesia and Hemiatrophy of the Tongue Michael J
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES Hemiageusia, Hemianaesthesia and Hemiatrophy of the Tongue Michael J. Strong and John H. Noseworthy ABSTRACT: A patient with a submandibular gland carcinoma was found clinically to have a unilateral chorda tympani, lingual and hypoglossal nerve deficit. This unique neurological entity of loss of taste sensation of one-half of the tongue (hemiageusia), hemianaesthesia and hemiatrophy of the tongue, has not previously been reported. RESUME: L'hemiageusie, I'hemianesthesie, et I'hemiatrophie de la langue Description dun patient porteur d'un carcinome de la glande sous-maxillaire qui presentait cliniquement un deficit unilateral de la fonction des nerfs suivants: corde du tympan, lingual et hypoglosse. Cette entite neurologique unique consistant en une perte du sens du gout sur la moitie' de la langue (hemiageusie), une hemianesthesie et une hemiatrophie de la langue, n'a encore jamais 616 raPP°rt6e- can. J. Neurol. Sci. 1986: 13:109-110 Lesions of the floor of the mouth occasionally present with in association with ipsilateral chorda tympani and hypoglossal predominantly neurological manifestations. While the entity of nerve dysfunction is demonstrated by this instructive case. hemianaesthesia and hemiatrophy of the tongue with loss of While intra-axial infiltration or degenerative processes should taste sensation has not previously been described, an under be considered, the clear lack of accompanying brainstem dys standing of the regional anatomy of the submandibular fossa function and the striking peripheral nature of the trigeminal can lead to only one localization for the lesion in this triad. We involvement renders this anatomically improbable. Similarly, have studied a patient presenting with these findings who was consideration of the more commonly seen and well recognized found to have a submandibular gland carcinoma. -

Endoscopic Selective Neck Dissection in a Porcine Model
ORIGINAL ARTICLE Endoscopic Selective Neck Dissection in a Porcine Model David J. Terris, MD; Ashkan Monfared, BS; Adrian Thomas, BS; Neeraja Kambham, MD; Yamil Sa´enz, DVM Objective: To investigate the feasibility of accomplish- essary. The median operative time was 131 minutes (range, ing a selective neck dissection (SND) endoscopically. 95-235 minutes). The median estimated blood loss was 4 mL (range, 0-150 mL). The mean±SD specimen weight Study Design: Prospective, nonrandomized experi- was 42.9±8.3 g; the mean number±SD of nodes re- mental investigation in a porcine model. trieved from the neck specimen was 4.8±2.2, and the mean±SD maximal nodal dimension was 2.4±0.5 cm. The Methods: Unilateral endoscopic SNDs were performed in arterial PCO2 increased by an average of only 3.9 mm Hg Yorkshire pigs. A spacious operative pocket was devel- from the beginning to the end of the surgery; correspond- oped using a combination of hernia balloon expansion fol- ingly, the pH fell by only 0.02. There were no major com- lowed by low-pressure (4 mm Hg) carbon dioxide insuf- plications, and no animals had to be euthanized prior to flation. The sternomastoid muscle, thymus, submandibular the completion of the procedure. gland, lymph nodes, and fibrofatty tissue were removed in a procedure approximating a human SND. Data (opera- Conclusions: Endoscopic neck dissection in a por- tive time, blood loss, arterial blood gas values, weight of cine model can be accomplished with a combination the specimen, and complications) were prospectively re- of strategies to overcome the dilemma of creating and corded. -

Carotid Triangle
• The submandibular triangle (or submaxillary or digastric triangle) corresponds to the region of the neck immediately beneath the body of the mandible Boundaries and coverings • It is bounded: • above, by the lower border of the body of the mandible, and a line drawn from its angle to the mastoid process; • below, by the posterior belly of the Digastricus; in front, by the anterior belly of the Digastricus. • It is covered by the integument, superficial fascia, Platysma, and deep fascia, ramifying in which are branches of the facial nerve and ascending filaments of the cutaneous cervical nerve. • Its floor is formed by the Mylohyoideus anteriorly, and by the hyoglossus posteriorl revision Submandibular Triangle • The submandibular triangle is located underneath the body of the mandible. It contains the submandibular gland (salivary), and lymph nodes. The facial artery and vein also pass through this area. • The boundaries of the submandibular triangle are: • Superiorly – body of the mandible. • Anteriorly – anterior belly of the digastric muscle. • Posteriorly – posterior belly of the digastric muscle • Boundaries • anteroinferiorly: anterior belly of digastric • posteroinferiorly: posterior belly of digastric • base: mandible • floor: mylohyoid, hyoglossus and middle pharyngeal constrictor • roof: skin, superficial fascia, platysma and deep fascia • Contents • anterior part of the triangle contains the submandibular gland • posterior part of the triangle contains the lower part of the parotid gland • facial artery is deep to the submandibular -

Ministry of Health of Ukraine Ukrainian Medical Stomatolgical Academy
Ministry of Health of Ukraine Ukrainian Medical Stomatolgical Academy Methodical Instructions for independent work of students during the training for the practical studies Academic discipline Surgical stomatology Module № 6 The topic of the stadies Lymphadenitis. Adenophlegmons. Abscesses of №8 face, palate, alveololingual groove, hyoid area. Physical therapy in the treatment of inflammatory diseases of maxillofacial area. Phlegmons of submandibular, submental areas and pterygopalatine-mandibular space. Course V Faculty Foreign Students Training, Stomatological Poltava -2020 1. Relevance of the topic: One of the most urgent issues of dentistry nowadays are acute odontogenic inflammatory processes. Despite the development of new methods of treating purulent infection, the number of patients with inflammatory diseases has an aggressive tendency to increase, leading to difficult complications. They not only cause temporary disability of patients, but also, due to serious complications, can be fatal. Iflammation in the maxillofacial area is predominantly odontogenic, associated with pathological processes in the dentofacial segment, starting from complicated caries, difficult dentition, periodontitis, etc. Inflammatory processes of tonsillogenic, rhinogenic, hematogenous and others are also found in the maxillofacial area. 2. THE SPECIFIC AIMS: 2.1.To analize, to know the statistics, classification , characteristics of etiology and pathogenesis , clinical signs of inflammatory processes of the maxillofacial area . 2.2. To explain the diagnostic methods of surface odontogenic inflammatory processes of the maxillofacial area. 2.3. To suggest inspect a patient with superficial inflammatory odontogenic processes of maxillofacial area. 2.4. To classificate the odontogenic inflammation of the submandibular area. 2.5. To explain the theoretical and clinical research on the problem of odontogenic submandibular phlegmonous adenitis . -

Anterior Cervical Triangle the Anterior Triangle of the Neck Is Bounded By
Anterior Cervical Triangle The anterior triangle of the neck is bounded by 1. the anterior border of the SCM 2. the anterior midline of the neck. 3. the mandible. 1 Anterior Cervical Triangle The anterior triangle is subdivided into four smaller triangles for descriptive purposes 1. Submental triangle 2. Submandibular (digastric) triangle 3. Carotid triangle 4. Muscular (omotracheal) triangle 2 The submental triangle inferior to the chin is an unpaired suprahyoid area bounded by : 1. the body of the hyoid bone inferiorly 2. the right and left anterior bellies of the digastric muscles laterally. 3 The submental triangle The floor of the submental triangle is formed by the two mylohyoid muscles, which meet in a median fibrous raphe. The apex of the submental triangle is at the mandibular symphysis The base is formed by the hyoid bone. 4 The submental triangle This triangle contains: 1. several small submental lymph nodes. 2. small veins that unite to form ' the anterior jugular vein. 5 6 7 The submandibular triangle It is a glandular area between the inferior border of the mandible and the anterior and posterior bellies of the digastric and the stylohyoid muscle Above by the lower border of the mandible Some people refer to it as the "digastric triangle." The floor of the submandibular triangle is formed by the mylohyoid muscle, hyoglossus muscle, and middle constrictor of the pharynx. 8 Anterior of the triangle contains the submandibular salivary gland the facial deep to the gland The vein and the submandibular LN superficial to the gland The hypoglossal nerve runs on the hypoglossal muscle deep to the gland Maylohyiod nerve and vessel runs inferior surface of this muscle 9 10 Posterior par of the triangle lies the carotid sheath, with the carotid arteries, internal jugular vein, vagus nerve The lower part of the parotid gland projects into the triangle.