Master Thesis Gastrointestinal Parasites in Sympatric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Samer I Østerdalen? En Studie Av Etnisitet I Jernalderen
Samer i Østerdalen? En studie av etnisitet i jernalderen og middelalderen i det nordøstre Hedmark Jostein Bergstøl Universitetet i Oslo 2008 Innholdsfortegnelse: Kapittel 1.................................................................................................................................... 1 1.1. Innledning........................................................................................................................ 1 1.1.1. Overordnede problemstillinger ................................................................................ 1 1.1.2. Presentasjon av studieområdet ................................................................................. 2 1.1.3. Noen begrepsavklaringer.......................................................................................... 3 1.2. Forskningshistorie .......................................................................................................... 3 1.2.1. ”Norrønt” og ”samisk” i forhistorien ....................................................................... 3 1.2.2. Samisk (for)historie.................................................................................................. 5 1.2.3. ”Den Norske Jernalderen”........................................................................................ 6 1.2.4. Fangstfolk i sør.........................................................................................................9 1.2.5. Jernalderen i Østerdalsområdet .............................................................................. 11 -

Innkalling Til Årsmøte
INNKALLING TIL ÅRSMØTE Sted: Vollan gård Tid: 19. mars 2019 klokken 1930 Sakliste: 1. Åpning 2. Årsmeldinger 3. Regnskap 4. Innkomne saker Forslag til vedtektsending 5. Valg 6. Budsjett Styrets medlemmer: Leder: Trond Livden, Rute 603, 2500 Tynset Nestleder: Ivar Næverdal, Innset, 7398 Rennebu Styremedlem: Stein Grue, 2512 Kvikne Styremedlem: Geir Atle Bobakk, Rute 603, 2500 Tynset Styremedlem: Henning Brevad 2512 Kvikne 1. Varamedlem: Audun Kregnes, 2500 Tynset 2. Varamedlem: Magne Thorleiv Rønning, 2512 kvikne Valgkomiteens medlemmer: Leder: Jan Karstensen, 2500 Tynset Medlem: Arnstein Solem, 2512 Kvikne Medlem: Conrad Schärer, 2512 Kvikne På valg i år: Leder: Trond Livden Styremedlem: Henning Brevad, 2512 Kvikne Styremedlem: Ivar Næverdal, Innset, 7398 Rennebu Varamedlem: Audun Kregnes, 2500 Tynset Valgkomité: Jan Karstensen, 2500 Tynset Møteleder: Steinar Munkhaugen, 2500 Tynset Vara møteleder: Arne Ingvar Nymoen, 2512 Kvikne Kvikne Utmarksråd Årsmelding for Kvikne Utmarksråd for 2018 05.03.2019 2 Kvikne Utmarksråd Forsidebilde: Rusefiske for innlegg av rogn i Kvikne klekkeri høsten 2018. INNHOLD ÅRSMELDING FRA LEDER ........................................................................................... 3 ÅRSMELDING FRA SMÅVILTANSVARLIG ................................................................... 4 ÅRSMELDING FRA VILLREINANSVARLIG................................................................... 6 ÅRSMELDING FRA FISKEANSVARLIG ........................................................................ 8 ÅRSMELDING FRA ELG- -
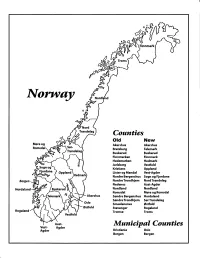
Norway Maps.Pdf
Finnmark lVorwny Trondelag Counties old New Akershus Akershus Bratsberg Telemark Buskerud Buskerud Finnmarken Finnmark Hedemarken Hedmark Jarlsberg Vestfold Kristians Oppland Oppland Lister og Mandal Vest-Agder Nordre Bergenshus Sogn og Fjordane NordreTrondhjem NordTrondelag Nedenes Aust-Agder Nordland Nordland Romsdal Mgre og Romsdal Akershus Sgndre Bergenshus Hordaland SsndreTrondhjem SorTrondelag Oslo Smaalenenes Ostfold Ostfold Stavanger Rogaland Rogaland Tromso Troms Vestfold Aust- Municipal Counties Vest- Agder Agder Kristiania Oslo Bergen Bergen A Feiring ((r Hurdal /\Langset /, \ Alc,ersltus Eidsvoll og Oslo Bjorke \ \\ r- -// Nannestad Heni ,Gi'erdrum Lilliestrom {", {udenes\ ,/\ Aurpkog )Y' ,\ I :' 'lv- '/t:ri \r*r/ t *) I ,I odfltisard l,t Enebakk Nordbv { Frog ) L-[--h il 6- As xrarctaa bak I { ':-\ I Vestby Hvitsten 'ca{a", 'l 4 ,- Holen :\saner Aust-Agder Valle 6rrl-1\ r--- Hylestad l- Austad 7/ Sandes - ,t'r ,'-' aa Gjovdal -.\. '\.-- ! Tovdal ,V-u-/ Vegarshei I *r""i'9^ _t Amli Risor -Ytre ,/ Ssndel Holt vtdestran \ -'ar^/Froland lveland ffi Bergen E- o;l'.t r 'aa*rrra- I t T ]***,,.\ I BYFJORDEN srl ffitt\ --- I 9r Mulen €'r A I t \ t Krohnengen Nordnest Fjellet \ XfC KORSKIRKEN t Nostet "r. I igvono i Leitet I Dokken DOMKIRKEN Dar;sird\ W \ - cyu8npris Lappen LAKSEVAG 'I Uran ,t' \ r-r -,4egry,*T-* \ ilJ]' *.,, Legdene ,rrf\t llruoAs \ o Kirstianborg ,'t? FYLLINGSDALEN {lil};h;h';ltft t)\l/ I t ,a o ff ui Mannasverkl , I t I t /_l-, Fjosanger I ,r-tJ 1r,7" N.fl.nd I r\a ,, , i, I, ,- Buslr,rrud I I N-(f i t\torbo \) l,/ Nes l-t' I J Viker -- l^ -- ---{a - tc')rt"- i Vtre Adal -o-r Uvdal ) Hgnefoss Y':TTS Tryistr-and Sigdal Veggli oJ Rollag ,y Lvnqdal J .--l/Tranbv *\, Frogn6r.tr Flesberg ; \. -

Forollhogna Og Bygdene
Jon J. Meli Forollhogna og bygdene Hogna sett fra nord. Foto: Forfatteren. 14. september ble Forollhogna Nasjonal- park offisielt åpnet av Miljøvernminister Børge Brende og Fylkesmennene Kåre Gjøn- nes og Sigbjørn Johnsen. På grunn av stridt vær måtte deler av arrangement skje i Dalsbygda Samfunnshus, før en kunne stif- te nærmere bekjentsskap med Nasjonalpar- ken og Såtthaugen. Jon J. Meli var den som orienterte statsråden og de andre frammøtte om Forollhognaområdet. Forollhogna nasjonalpark er åpnet. Fylkesmann Sigbjørn Johnsen, statsråd Børge Brende og fylkesmann Kåre Gjønnes. Foto: Forfatteren. 18 Årbok for Nord-Østerdalen 2002 Mange av dere har vel den siste tida arten fra området. Sjøl med totalfred- fulgt med i et fjernsynsprogram med ning fra 1930, er det bare sporadisk en tittelen: Villdyra kommer. Serien vart for kan møte denne fjellets sjarmør. Mest ca tre uker siden avslutta og en av de tallrik i Norge i dag er den i Børgefjell siste kommentarene jeg hørte fra pro- – vår annen nasjonalpark som vart gramlederen, var at ingen arter hadde oppretta i 1963. ”evig” liv! Dette fikk meg til å tenke på en art – en art som var svært tallrik i Foroll- 1000 års aktivitet i området! hognafjella inntil for 70-80 år sida; Ja dette var kanskje ei rar innledning fjellreven, men som vi de siste 8-10 åra når en skal fortelle om ”Forollhogna ikke har sikre observasjoner på om gjennom tidene”? Men ser vi på området den fortsatt lever her. Fjellreven, den i historisk perspektiv, er det vel net- har for øvrig mange navn, kan nevne topp de ville dyra som har brukt områ- et par; melrakke og vehund, har nok det mest og som burde ha sitt rettmes- gjennom tusenår vært en av karakter- sige krav på oppmerksomhet i en slik artene her i fjella, det vitner alle dens anledning! Men jeg skal likevel fortelle bosteder om, nedtegnelser i samtaler dere andre småtrekk fra området og, med eldre fjellfolk og det tyder navn- slik jeg finner det interessant. -

Forvaltningsplan for Kvikne Østre Statsallmenning
Forvaltningsplan for Kvikne Østre statsallmenning Av Kvikne fjellstyre April 2015 SAMMENDRAG 3 1. NATURGRUNNLAGET 4 1.1. Beliggenhet 4 1.1.1. Stedsbeskrivelse 4 1.1.2. Grensebeskrivelse 4 1.2. Topografi 5 1.3. Vegetasjon 5 1.4. Fisk 5 1.5. Vilt 6 1.5.1. Hjortevilt 6 1.5.2. Hønsefugl 6 1.5.3. Gnagere 8 1.5.4. Fugleliv tilknyttet våtmark 8 1.5.5. Rovfugl 8 1.5.6. Rovdyr 8 1.5.7 Smårovvilt 8 1.6. Landbruk 9 1.7. Hytter og annen bebyggelse 9 1.8. Vei, sti og løypenett 9 1.9. Kulturminner 10 1.10. Økonomi 10 2. PLANSITUASJON 11 2.1. Foreliggende planer i området 11 3. SANNSYNLIG UTVIKLING 11 3.1. Framtidige ønsker om areal for utbygging 11 3.2. Organisert utnytting av området av fremmede aktører eller annen næringsvirksomhet 11 3.3. Sannsynlige endringer i bygdefolkets bruk 12 3.4. Sannsynlige konfliktområder og mulige samarbeidsområder 12 3.5. Mulige nye inntektsområder 12 4. PLANDEL 13 4.1. Fiskeforvaltning og fiskestell 13 2 4.1.1. Fiskekortsamarbeid 13 4.1.2. Utsetting av oppdrettsfisk 13 4.1.3. Overvåkning 13 4.2. Viltforvaltning og viltstell 13 4.2.1. Villrein 13 4.2.2. Rype 14 4.2.3. Rovfugl 14 4.2.4. Rovdyr 14 4.3. Tilrettelegging for alminnelig ferdsel 15 4.4. Tilrettelegging for camping 15 4.5. Tilrettelegging for virksomhet tilknytta landbruket 15 5. LITTERATUROVERSIKT 15 SAMMENDRAG Statsallmenningen forvaltes av Kvikne fjellstyre, med to medlemmer fra Rennebu kommune og tre fra Tynset kommune. -

Exploring 21St Century Norway
Exploring 21st Century Norway Join the Gudbrandsdalslag of America as they travel through Norway in the steps of with Gudbrandsdalslag of America their ancestors and discover how ancient June 19–July 2, 2016 traditions have helped shape the lives of those living in Norway in the 21st Century! Dombås Bøverdal Vinstra Skjolden Lillehammer Voss Flåm Lofthus NORWAY Oslo Stavanger DAY 1 SUNDAY, JUNE 19 DAY 5 THURSDAY, JUNE 23 [B,D] ITINERARY INCLUDES: Mpls/St Paul—Stavanger: Depart the USA on your Lofthus—Voss—Flåm: Breakfast at hotel. Drive transatlantic flight. To request alternative dates or to Voss. Continue by train to Myrdal and change • Roundtrip airfare from Mpls / St. Paul, for estimated airfare supplements from other US to the Flåm Railway. Descend the Flåm Valley, including taxes, fees & fuel surcharge cities, please note on application. dropping 2800 feet within 13 miles to the idyllic fjord • 12 nights accommodations, 1st class/superior community of Flåm. Cruise the dramatic Aurlandfjord tourist class, double occupancy DAY 2 MONDAY, JUNE 20 [D] and Nærøyfjord from Flåm to Gudvangen. Drive to Stavanger: Arrive at Sola Airport Stavanger. Stalheim and descend the 13 thrilling hairpin bends • Daily breakfast [B], 5 lunches [L] and 11 Transfer to a hotel in city center. Balance of the day of the Stalheim Road. Dinner and overnight at dinners [D] free to relax and adjust to the time change. Welcome Fretheim Hotel. • 1st-class touring coach dinner and overnight at Clarion Hotel Stavanger. DAY 6 FRIDAY, JUNE 24 [B,D] • City sightseeing tour in Stavanger DAY 3 TUESDAY, JUNE 21 [B,D] Flåm—Skjolden: Breakfast at hotel. -

Skadefellingstillatelse På Ulv I Tynset Kommune – Forlenget Virketid
Vår dato: Vår ref: 28.05.2019 2019/11601 Deres dato: Deres ref: Saksbehandler, innvalgstelefon Tynset og Alvdal kommuner, landbruk og miljø Rannveig Helgesen, 62 55 11 75 2560 ALVDAL e-post: [email protected] Skadefellingstillatelse på ulv i Tynset kommune – forlenget virketid Vi viser til Fylkesmannens brev av 23. mai med skadefellingstillatelse på en ulv, og til telefonsamtale med leder i Kvikne Vestfjell beitelag den 27. og 28. mai hvor det muntlig ble søkt om forlengelse av skadefellingstillatelsen. Fylkesmannen ga muntlig tillatelse til forlengelse før skadefellingstillatelsen gikk ut kl. 12:00 i dag. Den 22. mai informerte Statens naturoppsyn Fylkesmannen om at de var ute og sjekket spor i Tunndalen i Tynset kommune, og konklusjonen på sporene ble satt til «antatt ulv» ut i fra skrittlengde og sporstørrelse. Beitelagene i Kvikne Øst- og Vestfjell søkte om skadefellingstillatelse, og det ble gitt tillatelse til skadefelling fram til 28. mai kl. 12:00. De siste dagene er det opplyst at det er gjort noen syns- og sporobservasjoner, uten at det er verifisert at disse observasjonene er dokumentert ulv. Det er dermed noe usikkerhet knyttet til om ulven har vandret ut av området eller om den fortsatt oppholder seg i beiteområdene. Med bakgrunn i observasjoner og hendelsesforløp, og ut fra områdets betydning som beitemark, samt potensialet for framtidige skader dersom ulven fortsatt oppholder seg innenfor beiteområdene til Kvikne Vestfjell og Kvikne Østfjell beitelag, samt tilgrensende beiteområder, forlenger Fylkesmannen virketiden for skadefellingstillatelsen i området. Fylkesmannen fatter vedtak om forlenget virketid for skadefellingstillatelsen gitt ved brev av 23.05.2019. Tillatelsen gis med samme vilkår, med unntak av punkt 2. -

Historisk Oversikt Over Endringer I Kommune- Og Fylkesinndelingen
99/13 Rapporter Reports Dag Juvkam Historisk oversikt over endringer i kommune- og fylkesinndelingen Statistisk sentralbyrå • Statistics Norway Oslo–Kongsvinger Rapporter I denne serien publiseres statistiske analyser, metode- og modellbeskrivelser fra de enkelte forsknings- og statistikkområder. Også resultater av ulike enkeltunder- søkelser publiseres her, oftest med utfyllende kommentarer og analyser. Reports This series contains statistical analyses and method and model descriptions from the different research and statistics areas. Results of various single surveys are also pub- lished here, usually with supplementary comments and analyses. © Statistisk sentralbyrå, mai 1999 Ved bruk av materiale fra denne publikasjonen, vennligst oppgi Statistisk sentralbyrå som kilde. ISBN 82-537-4684-9 ISSN 0806-2056 Standardtegn i tabeller Symbols in tables Symbol Tall kan ikke forekomme Category not applicable . Emnegruppe Oppgave mangler Data not available .. 00.90 Metoder, modeller, dokumentasjon Oppgave mangler foreløpig Data not yet available ... Tall kan ikke offentliggjøres Not for publication : Emneord Null Nil - Kommuneinndeling Mindre enn 0,5 Less than 0.5 of unit Fylkesinndeling av den brukte enheten employed 0 Kommunenummer Mindre enn 0,05 Less than 0.05 of unit Sammenslåing av den brukte enheten employed 0,0 Grensejusteringer Foreløpige tall Provisional or preliminary figure * Brudd i den loddrette serien Break in the homogeneity of a vertical series — Design: Enzo Finger Design Brudd i den vannrette serien Break in the homogeneity of a horizontal series | Trykk: Statistisk sentralbyrå Rettet siden forrige utgave Revised since the previous issue r Sammendrag Dag Juvkam Historisk oversikt over endringer i kommune- og fylkesinndelingen Rapporter 99/13 • Statistisk sentralbyrå 1999 Publikasjonen gir en samlet framstilling av endringer i kommuneinndelingen 1838 - 1998 og i fylkesinndelingen 1660 - 1998. -

HA-Sprinten 2018 Tdh-1 Startliste Budor Skistadion 15.12.2018
HA-Sprinten 2018 TdH-1 Startliste Budor Skistadion 15.12.2018 G 11 år Start nr. Navn Klubb Tid Brikkenr 1 Ludvig Bjerregård OtterholmRaufoss IL Langrenn 09:00:00 3907953 2 Krisander Kørra Jømna/Heradsbygd Langrenn 09:00:15 3 Sondre Laforce Hamar SK 09:00:30 4 Mathias Skeie-Nilsen Strandbygda IL 09:00:45 5 Magnus Vestmo Almåsbak Grue IL 09:01:00 3928009 6 Henrik Lindbakken Strandbygda IL 09:01:15 7 Markus Børke MjøsSki 09:01:30 8 Ludvik Hagen Myhrer Strandbygda IL 09:01:45 9 Ludvik Rustadbakken Kjendlie Hamar SK 09:02:00 4006649 10 Emil Kolloen Ringsaker IF 09:02:15 11 Martin Moslet Evensen Raufoss IL Langrenn 09:02:30 3825023 12 Oliver Stangstuen Kjeldsberg Vind IL 09:02:45 3988482 13 Henrik Sebastian Jensrud Hamar SK 09:03:00 3988573 14 Sondre Borgen Østre Toten Skilag 09:03:15 3936911 15 Sivert Dragerengen Raufoss IL Langrenn 09:03:30 16 Oliver Kjellaas Strandbygda IL 09:03:45 17 Simen Midtseim Halvorsrud Oppstad IL 09:04:00 18 Erling Bogsti Moelven IL 09:04:15 19 Peder Hagen Strandbygda IL 09:04:30 3933033 20 Vetle Østlien Evensen Vind IL 09:04:45 3798022 21 Magnus Benjaminsen MjøsSki 09:05:00 3652997 22 Martin Kaulum Hamar SK 09:05:15 Fullførte:0 Påmeldte:22 Startende: 22 J 11 år Start nr. Navn Klubb Tid Brikkenr 25 Frida Rasmussen Jømna/Heradsbygd Langrenn 09:06:00 26 Elise Stangstuen Kjeldsberg Vind IL 09:06:15 3988219 27 Camilla Nordvold Lunde Åslia Skilag 09:06:30 28 Hedda Faraasen Alme Strandbygda IL 09:06:45 3996360 29 Ella Wiken Vang Skiløperforening 09:07:00 30 Olaug Bjørklund Nord-Odal IL 09:07:15 31 Maria Hagen Hamar SK 09:07:30 32 Lilli-Kristine Lagmandsveen MjøsSki 09:07:45 3819125 33 Julie Haug Lundby Østre Toten Skilag 09:08:00 34 Emilie Ruud Lia Granli IL 09:08:15 3999729 Fullførte:0 Påmeldte:10 Startende: 10 G 12 år Start nr. -
Download the English Version of the Tourist Guide
TOURISTGUIDE FOR HOLTÅLEN – RØROS – OS – TOLGA – TYNSET – ALVDAL Be captured TOURIST INFORMATION IN THE RØROS REGION Please visit our region’s tourist information centres WELCOME! for available attractions and activities. It is with great pride that we welcome you to our region. In this guide you will find information about a wide range OPEN ALL YEAR SUMMER OPEN Approx 20. June – 20. August of activities throughout the 6 counties Holtålen, Røros, Os, Opening hours Monday to Friday: 09.00 to 15.00 Tolga, Tynset and Alvdal and the surrounding mountain RØROS HOLTÅLEN wilderness and its historical setting. We can offer you a Røros Tourist Information Eidet rest stop packed cultural calendar throughout the year, world class A: Peder Hiortsgt. 2. A: Ålen T: +47 72 41 00 00 T: +47 913 38 298. local food, historical landmarks, Røros, one of UNESCO’s world heritage sites, but most of all, a warm welcome. OS HOLTÅLEN Tourist Information next to Best Gammelgården A: Brutippen 9 A: Haltdalen T: + 47 970 54 023 T: +47 906 58 772 We hope you have a wonderful stay! TOLGA TOLGA Tolga Tourist Information, Vingelen Tourist Information, YX Tolga, Caltex café Bunåva A: Tolga A: Vingelen T: +47 62 49 42 80. T: +47 479 21 187 Tove R. Martens, Director of tourism TYNSET TYNSET Tynset Tourist Information Tourist Information next to Circle A: Tynset Rådhus K - Kvikne T: + 47 62 48 52 49 A: Situated by the main road, RV3. T: + 47 62 48 5249 SHARE YOU EXPERIENCES ALVDAL Use #destinationroros and #destinasjonrøros on Instagram. Alvdal Tourist Information A: Aukrustsenteret. -

WEEKLY Formerly
Moods of Norway This week on En Erfaren manns råd er mer verd enn en liten opens in Beverly Hills skytshelgen. norway.com Business - Ordtak Sports > Page 4 TIME DATED MATERIAL — DO NOT DELAY (Periodicals postage paid at Seattle, WA) Norwegian American Formerly WEEKLY Formerly Vol. 120, No. 22 June 5, 2009 7301 Fifth Avenue NE Suite A, Seattle, WA 98115 Tel (800) 305-0217 • www.norway.com $1.50 per copy Online News Dateline Oslo The Wergeland Centre inaugurated Cancer risk for child Crown Princess Mette- survivors Marit was present at Survivors of childhood cancer the inauguration of the have a higher life-long risk European Wergeland of developing a new form of Centre in Oslo on Friday the disease, a study shows. May 26. The centre shall A team from the Institute promote education for of Cancer Epidemiology in intercultural understanding, Copenhagen studied 47,679 human rights and people who were diagnosed democratic citizenship with cancer before the age of 20, between 1943 and 2005. BERIT HESSEN They were drawn from the Managing Editor cancer registries of Denmark, Finland, Iceland, Norway and Sweden. (BBC News) The Centre was established by Norway in cooperation with the Norway increases aid to Council of Europe in 2008. Zimbabwe Henrik Arnold Werge- Norway is increasing its land (1808 – 1845) was a romantic Norwegian poet and an important aid to Zimbabwe by NOK historian. He worked for the inde- 58 million. Minister of pendence of all nations, was an ad- International Development vocate for democracy, and an eager Erik Solheim says the money defender of freedom of faith and will be used to help the people freedom of expression. -

Etternavn Fornavn Poststed Daffinrud Terje Rennebu 1 Begge Felt
Søker Søker for Etternavn Fornavn Poststed antall jegere Jaktfelt og periode Daffinrud Terje Rennebu 1 Begge felt, 10.-24.september Austvik Kristin Lund Kvikne 1 Begge felt, 10.-24.september Fossum Kenth Egil Kvikne 1 Begge felt, 10.-24.september Heilmann Rainer Kvikne 1 Begge felt, 10.-24.september Mælen Arne Tynset 1 Begge felt, 10.-24.september Nygård Kåre Inge Rennebu 1 Begge felt, 10.-24.september Olsbakk Bjørn Arvid Kvikne 1 Begge felt, 10.-24.september Skullerud Esten Sødal Kvikne 1 Begge felt, 10.-24.september Stai Ragnhild Kvikne 1 Begge felt, 10.-24.september Stenrud John Kvikne 1 Begge felt, 10.-24.september Stenrud Tone Kvikne 1 Begge felt, 10.-24.september Strand Pål Kvikne 1 Begge felt, 10.-24.september Stuen Jan Erik Rennebu 1 Begge felt, 10.-24.september Bergstrøm Jan Fredrik Ås 1 1.periode nordre Nyborg Trond Leif Heggedal 1 Nilsen Stig Løten 3 Austberg Per Erik Heimdal 1 Amundsen Roald Bødalen 3 1.periode søndre Ørvim Tom Langhus 1 Kristiansen Jan Morten Sarpsborg 1 Stø Andreas Fåberg 1 Hagen Stein Ole Hakadal 1 Nordli Andre Kjeller 3 2. periode nordre Hagen Stein Ole Hakadal 1 Langsnes Espen Eide 2 Rosmo Stig Heimdal 2 Sperling Bjørn Tore Sellebakk 1 2. periode søndre Ødegård Tron Hagan 1 Finnbråten Bjørn D. Kolsås 2 Heggedal Trine Sandefjord 2 Sellgren Nils Trondheim 1 Berner Fredrik Gran 1 Stykket Andres Tvedestrand 3 3. periode nordre Wikdahl Petter Trondheim 1 Olsen Brynjulf Flage Slependen 3 Waagbø Arne Oppegård 1 3. periode søndre Dammen Lars Slependen 3 Gravset Øyvind Rena 4 Henriksen Astrid E.