Anti-Microbial Activity of Ilavu Ver Chooranam & The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

India Progressive Writers Association; *7:Arxicm
DOCUMENT RESUME ED 124 936 CS 202 742 ccpp-.1a, CsIrlo. Ed. Marxist Influences and South Asaan li-oerazure.South ;:sia Series OcasioLal raper No. 23,Vol. I. Michijar East Lansing. As:,an Studies Center. PUB rAIE -74 NCIE 414. 7ESF ME-$C.8' HC-$11.37 Pius ?cstage. 22SCrIP:0:", *Asian Stud,es; 3engali; *Conference reports; ,,Fiction; Hindi; *Literary Analysis;Literary Genres; = L_tera-y Tnfluences;*Literature; Poetry; Feal,_sm; *Socialism; Urlu All India Progressive Writers Association; *7:arxicm 'ALZT:AL: Ti.'__ locument prasen-ls papers sealing *viithvarious aspects of !',arxi=it 2--= racyinfluence, and more specifically socialisr al sr, ir inlia, Pakistan, "nd Bangladesh.'Included are articles that deal with _Aich subjects a:.the All-India Progressive Associa-lion, creative writers in Urdu,Bengali poets today Inclian poetry iT and socialist realism, socialist real.Lsm anu the Inlion nov-,-1 in English, the novelistMulk raj Anand, the poet Jhaverchan'l Meyhani, aspects of the socialistrealist verse of Sandaram and mash:: }tar Yoshi, *socialistrealism and Hindi novels, socialist realism i: modern pos=y, Mohan Bakesh andsocialist realism, lashpol from tealist to hcmanisc. (72) y..1,**,,A4-1.--*****=*,,,,k**-.4-**--4.*x..******************.=%.****** acg.u.re:1 by 7..-IC include many informalunpublished :Dt ,Ivillable from othr source r.LrIC make::3-4(.--._y effort 'c obtain 1,( ,t c-;;,y ava:lable.fev,?r-rfeless, items of marginal * are oft =.ncolntered and this affects the quality * * -n- a%I rt-irodu::tior:; i:";IC makes availahl 1: not quali-y o: th< original document.reproductiour, ba, made from the original. -

The Day of the African Child 30 Years On, Where Are We Now? Page 2
The Day of the African Child 30 years on, where are we now? Page 2: Adelaide Benneh Prempeh Advancing the Welfare of children in Ghana Page 18: Maria Mbeneka Child Marriage Page 22: Presentation Judge Fatoumata Dembélé Page 23: Diarra – Mali Euridce Baptista – Angola Page 24: CLA Role of the Law in Eliminating Child Marriage 2/10/21 ADVANCING THE WELFARE OF CHILDREN IN GHANA. Presented By: Adelaide Benneh Prempeh Managing Partner B&P ASSOCIATES 1 OVERVIEW u GHANAIAN HISTORICAL CONTEXT & BACKGROUND. u THE CHILD & FAMILY PROTECTION SYSTEM IN GHANA. u LEGAL FRAMEWORK. u LEGISLATIVE REFORM IN GHANA’S ADOPTION LAW. u ADVANCING CHILDREN’S RIGHTS FOR SUSTAINABLE DEVELOPMENT- EDUCATION. 2 1 2/10/21 I. GHANAIAN HISTORICAL CONTEXT & BACKGROUND 3 In the Ghanaian context “Best interests of the Child” will includes cultural perspective and respect for customary structure. Ø Extended family environment. Ø Benefits: Children are allowed to remain in a familiar environment, still connected with their natural family; safety net for children to receive support within family and community. Ø Challenges: Lack of an enforced legal regime to govern this type of “informal family arrangement” for more vulnerable children, as well as the inherent difficulty in monitoring such social arrangements, lends itself to corporal punishment, domestic violence, sexual abuse, sexual violence and exploitation, ritual servitude (Trokosi). 4 2 2/10/21 II. CHILD & FAMILY PROTECTION SYSTEM IN GHANA. 5 2013 International Social Service Report. Ø Weaknesses in the alternative care system/ fosterage. Ø Open adoption of children. Ø Child trafficking. 6 3 2/10/21 Technical Committee Recommendations: Ø Robust child protection framework; Ø Strong regulatory and supervisory institutions; Ø Licensing and accreditation of agencies; Ø Financing of residential facilities; and Ø Comprehensive Child and Family Framework. -
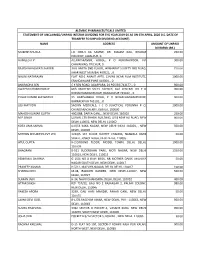
APL Details Unclaimed Unpaid Interim Dividend F.Y. 2019-2020
ALEMBIC PHARMACEUTICALS LIMITED STATEMENT OF UNCLAIMED/UNPAID INTERIM DIVIDEND FOR THE YEAR 2019‐20 AS ON 6TH APRIL, 2020 (I.E. DATE OF TRANSFER TO UNPAID DIVIDEND ACCOUNT) NAME ADDRESS AMOUNT OF UNPAID DIVIDEND (RS.) MUKESH SHUKLA LIC CBO‐3 KA SAMNE, DR. MAJAM GALI, BHAGAT 200.00 COLONEY, JABALPUR, 0 HAMEED A P . ALUMPARAMBIL HOUSE, P O KURANHIYOOR, VIA 900.00 CHAVAKKAD, TRICHUR, 0 RAJESH BHAGWATI JHAVERI 30 B AMITA 2ND FLOOR, JAYBHARAT SOCIETY 3RD ROAD, 750.00 KHAR WEST MUMBAI 400521, , 0 NALINI NATARAJAN FLAT NO‐1 ANANT APTS, 124/4B NEAR FILM INSTITUTE, 1000.00 ERANDAWANE PUNE 410004, , 0 ANURADHA SEN C K SEN ROAD, AGARPARA, 24 PGS (N) 743177, , 0 900.00 SWAPAN CHAKRABORTY M/S MODERN SALES AGENCY, 65A CENTRAL RD P O 900.00 NONACHANDANPUKUR, BANACKPUR 743102, , 0 PULAK KUMAR BHOWMICK 95 HARISHABHA ROAD, P O NONACHANDANPUKUR, 900.00 BARRACKPUR 743102, , 0 JOJI MATHEW SACHIN MEDICALS, I C O JUNCTION, PERUNNA P O, 1000.00 CHANGANACHERRY, KERALA, 100000 MAHESH KUMAR GUPTA 4902/88, DARYA GANJ, , NEW DELHI, 110002 250.00 M P SINGH UJJWAL LTD SHASHI BUILDING, 4/18 ASAF ALI ROAD, NEW 900.00 DELHI 110002, NEW DELHI, 110002 KOTA UMA SARMA D‐II/53 KAKA NAGAR, NEW DELHI INDIA 110003, , NEW 500.00 DELHI, 110003 MITHUN SECURITIES PVT LTD 1224/5 1ST FLOOR SUCHET CHAMBS, NAIWALA BANK 50.00 STREET, KAROL BAGH, NEW DELHI, 110005 ATUL GUPTA K‐2,GROUND FLOOR, MODEL TOWN, DELHI, DELHI, 1000.00 110009 BHAGRANI B‐521 SUDERSHAN PARK, MOTI NAGAR, NEW DELHI 1350.00 110015, NEW DELHI, 110015 VENIRAM J SHARMA G 15/1 NO 8 RAVI BROS, NR MOTHER DAIRY, MALVIYA 50.00 -

THE RECORD NEWS ======The Journal of the ‘Society of Indian Record Collectors’ ------ISSN 0971-7942 Volume: Annual - TRN 2011 ------S.I.R.C
THE RECORD NEWS ============================================================= The journal of the ‘Society of Indian Record Collectors’ ------------------------------------------------------------------------ ISSN 0971-7942 Volume: Annual - TRN 2011 ------------------------------------------------------------------------ S.I.R.C. Units: Mumbai, Pune, Solapur, Nanded and Amravati ============================================================= Feature Articles Music of Mughal-e-Azam. Bai, Begum, Dasi, Devi and Jan’s on gramophone records, Spiritual message of Gandhiji, Lyricist Gandhiji, Parlophon records in Sri Lanka, The First playback singer in Malayalam Films 1 ‘The Record News’ Annual magazine of ‘Society of Indian Record Collectors’ [SIRC] {Established: 1990} -------------------------------------------------------------------------------------------- President Narayan Mulani Hon. Secretary Suresh Chandvankar Hon. Treasurer Krishnaraj Merchant ==================================================== Patron Member: Mr. Michael S. Kinnear, Australia -------------------------------------------------------------------------------------------- Honorary Members V. A. K. Ranga Rao, Chennai Harmandir Singh Hamraz, Kanpur -------------------------------------------------------------------------------------------- Membership Fee: [Inclusive of the journal subscription] Annual Membership Rs. 1,000 Overseas US $ 100 Life Membership Rs. 10,000 Overseas US $ 1,000 Annual term: July to June Members joining anytime during the year [July-June] pay the full -
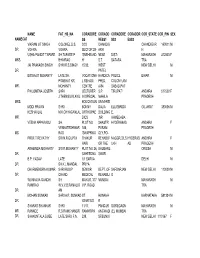
Professional-Address.Pdf
NAME FAT_HS_NA CORADDRE CORADD CORADDRE CORADDR COR_STATE COR_PIN SEX NAMECAT SS RESS1 SS2 ESS3 VIKRAM JIT SINGH COLONEL D.S. 3/33 CHANDIG CHANDIGAR 160011 M DR. VOHRA VOHRA SECTOR 28- ARH H USHA PANDIT TAPARE SH.TAPARE P 'SNEHBAND NEAR DIST- MAHARASH 412803 F MRS. BHIMRAO H' S.T. SATARA TRA JAI PRAKASH SINGH SHRI R.S.SINGH 15/32, WEST NEW DELHI M DR. PATEL BISWAJIT MOHANTY LATE SH. VOCATIONA HANDICA POLICE BIHAR M PRABHAT KR. L REHABI. PPED, COLONY,ANI MR. MOHANTY CENTRE A/84 SABAD,PAT PHILOMENA JOSEPH SHRI LECTURER S.P. TIRUPATI ANDHRA 517502 F J.THENGUVILAYIL IN SPECIAL MAHILA PRADESH MRS. EDUCATION UNIVERSI MODI PRAVIN SHRI BONNY DALIA ELLISBRIDG GUJARAT 380006 M KESHAVLAL M.K.CHHAGANLAL ORTHOPAE BUILDING E, MR. DICS ,NR AHMEDABA VEENA APPARASU SH. PLOT NO SHASTRI HYDERABAD ANDHRA F VENKATESHWAR 188, PURAM PRADESH MS. RAO 'SWAPRIKA' CLY,PO- PROF.T REVATHY SRI N.R.GUPTA THAKUR REHABI.F NAGGR,DILS HYDERAB ANDHRA F HARI OR THE UKH AD PRADESH ARABINDA MOHANTY SRI R.MOHANTY PLOT NO 24, BHUBANE ORISSA M DR. SAHEEDNA SWAR B.P. YADAV LATE 1/1 SARVA DELHI M DR. SH.K.L.MANDAL PRIYA DHARMENDRA KUMAR SHRI ROOP SENIOR DEPT. OF SAFDARJAN NEW DELHI 110029 M DR. CHAND MEDICAL REHABILI G WUNNAVA GANDHI SH MAYUR, 377 MUMBAI MAHARASH M RAMRAO W.V.V.B.RAMALIG V.P. ROAD TRA DR. AM MOHAN SUNKAD SHRI A.R. SUNKAD ST. HONAVA KARNATAKA 581334 M DR. IGNATIUS R SHARAS SHANKAR SHRI 11/17, PANDUR GOREGAON MAHARASH M MR. RANADE R.S.RAMCHANDR RAMKRIPA ANGWADI (E), MUMBAI TRA DR. -

HUM, He Assisted Olcott in the Collection of Funds For
P 0.-HISTORY OF EDUCATION 246. HUM, "Anagarika Dharmapala as an Educator."Journal of the National Education Societ ofCeylon.va.15, 1964. pp. 18 -32. When Dharmapala was a youth he came underthe influence of Colonel Olcott and FadameBlavatsky of the Theosophical Society who visited Ceylon in the188018. He assisted Olcott in the collection of funds forthe Buddhist educational movement.He later developed to be a great educational and socialreformer, and a religious leader.He worked in the fields of educational and social reform in both India and Ceylon.He was an admirer of the educational systems of Japan and the United States, and he urged India and Ceylon toestablish an educationalsystem that would make every child grow up to be an intelligentthinking individual with a consciousness active for the good of all beings. He set up schools to translate intopractice the idealshe had in mind.He emphasised the importance of technical education, especiallyinthe fields of agriculture and industry.He started several industrial schools and tried to wean students away from too bookish an education. He was a great believer in adult education, which he thought could be best promoted throughthe mass media. For this purpose, he started a Sinhala newspaper in Ceylon and anEnglish journal The lishabodhi Jou.1. which had a wide circulationin'IndlTW" o. Ceylon. 247. KARUNARATNE, V. T. G. "Educational changes in Ceylon." ---Ceylon Daily Nears. December 3, 1963. 1550 words. December 1, 1960 is significant in the history of education in Ceylon as the day on which one of the fetters of colonialism, namely the denominationalschool system, was shattered.The Britishencouraged missionary enterprise in Ceylon and were reluctant to enter the field of education. -

<3°SS*)^§@0 & 1&0 O S)Q5
<3 °SS*)^§@0 & 1 & 0 OS )Q5 THE CEYLON GOVERNMENT GAZETTE ff°2S» 1 2 ,1 4 9 — I 9 6 0 gjs£ 2 4 — 2 4 . 6 . 1 9 6 0 No. 12,149— FR ID A Y, JUNE 24, 1960 (Published by Authority) PART V-BOOK LIST, &c. (Separate paging is given to each language of every Part in order that it may he filed eeparately,) Statement of Books Printed in Ceylon and Registered under the Printers and Publishers Ordinance (Cap. 137), as amended by The Printers and Publishers (Amendment) Act, No. 28 of 1951, during the Quarter ended March 31, 1959 CONTRACTIONS : (a) The language in which the book is written ;(b) The name of the author, translator or editor of the book or any part thereof; (c) The subject; (d) The place of printing; (e) The place of publication ; (f) The name or firm of the printer ; (g) The name or firm of the publisher ; (h) The date of issue from the press ; (i) The number of pages ; (j) The size; (k) The first, second or other number of the edition; (1) The number of copies of which the edition consists ; (m) Whether the book is printed or lithographed; (n) The price at which the book is sold to the public ;(o) The name and ■residence of the proprietor of the copyright,or of any portion of the copyright. Quarter ended March 31,1959—First Quarter 1959 GENERAL WORKS GENERAL PERIODICALS 75175 Muthukumarath Thambiran—Ninaivu Malar 75270 Samastha Lanka Welanda Manthrana Sabhawa— (a) Tamil, (b) S. -

A Study of the History and Cult of the Buddhist Earth Deity in Mainland Southeast Asia
A Study of the History and Cult of the Buddhist Earth Deity in Mainland Southeast Asia A Thesis submitted in partial fulfillment of the requirements for the Degree of PhD in Religious Studies at the University of Canterbury by Elizabeth Guthrie University of Canterbury, Christchurch, New Zealand 2004 A Study of the History and Cult of the Buddhist Earth Deity in Mainland Southeast Asia Volume 1 Text Acknowledgements Far-ranging research projects like this inevitably depend on the generosity and assistance of many people. Among those who helped me find the earth deity in image and texts, or helped with translations, were: Ang Choulean, K. Aphaivong. Bandol Samnang, Olivier de Bernon, Didier Bertrand, Fran(,{ois Bizot, Robert L. Brown, Kaye Carter, Chuch Phoeun, Shayne Clarke, John Crocker, Denison University Art Gallery, Robert Didham, Wichai Eungpinichpong, Wilai Eungpinichpong, John Marston, Long Tbol, Des Sothy, Anthony Diller, Jacqueline Filliozat, Rolf Giebel, Hang Chan Sophea, Louis Gabaude, Pam Gutman, Anne Hansen, Huberta Hellendoorn, Hor Lath, Khy Sophal, Khyaw Tha Nyunt, Kuy Lath, Fran(,{ois Lagirarde, Lan Sunnary, Leng Kok An, Lim Yii Hang, Long Tbol, Meng Prang, Metropolitan Museum of Art, Mey Poeun, Museum flir Indische Kunst, Neou Chamrong, Norton Simon Museum, Ouk Ry, Anatole Peltier, Phaitun Dokbukaeo, Phon Sin, Phoung Soueng, Sommai Premchit, Thonevath Pou, Saveros Pou, Craig Reynolds, Waldemar Sailer, Sao Hso Hom, Peter Skilling, Frank Smith, Ven. Suthep Surapong, Donald Swearer, Thein Tun U, Serge Thion, Ashley Thompson, Vijinthanasarn Panya, U Aung Kyaing, U Myint Aung, RE. Vann Molyvann, John Weeks, Hiram W.Woodward, Jr. I received funding from the NZFUW, NZASIA and the University of Canterbury. -

The Gazette of the Democratic Socialist Republic of Sri Lanka
YS% ,xld m%cd;dka;s%l iudcjd§ ckrcfha .eiÜ m;%h The Gazette of the Democratic Socialist Republic of Sri Lanka wxl 2"023 – 2017 cqks ui 09 jeks isl=rdod – 2017'06'09 No. 2,023 – FRIDAY, JUNE 09, 2017 (Published by Authority) PART I : SECTION (I) – GENERAL (Separate paging is given to each language of every Part in order that it may be fi led separately) PAGE PAGE Proclamations, &c., by the President … — Government Notifi cations … … 757 Appointments, &c., by the President … 758 Price Control Orders … … — Appointments, &c., by the Cabinet of Ministers … 774 Central Bank of Sri Lanka Notices… … — Accounts of the Government of Sri Lanka … — Appointments, &c., by the Public Service Commission — Revenue and Expenditure Returns… … — Appointments, &c., by the Judicial Service Commission — Miscellaneous Departmental Notices … 834 Other Appointments, &c. … … — Notice to Mariners … … — Appointments, &c., of Registrars … — “Excise Ordinance” Notices … … — Note.– Local Authorities Elections (Amendment) Bill was published as a supplement to the Part II of the Gazette of the Democratic Socialist Republic of Sri Lanka of June 02, 2017. Laila Umma Deen Foundation (Incorporation) Bill was published as a supplement to the Part II of the Gazette of the Democratic Socialist Republic of Sri Lanka of June 02, 2017. IMPORTANT NOTICE REGARDING ACCEPTANCE OF NOTICES FOR PUBLICATION IN THE WEEKLY “GAZETTE” ATTENTION is drawn to the Notifi cation appearing in the 1st week of every month, regarding the latest dates and times of acceptance of Notices for publication in the weekly Gazettes, at the end of every weekly Gazette of Democratic Socialist Republic of Sri Lanka. -

Included in This Second Issue of a 3-Volume Series of Bibliographies with Abstracts Are 115 Items Dealing with Significant Mater
DOCUMENT RESUME ED 029 534 72 FL 001 318 By- Jayasuriya. J.E.. Comp. Ceylon Education Abstracts. January 1. 1960 to December 31. 1962. Volume 1.Number 2. University of Ceylon. Peradeniya. Spons Agency-Office of Education (DHEW). Washington, D.C. Pub Date 69 Note- 69p. EDRS Price MF-$0.50 HC-$3.55 Descriptors- Abstracts.*AnnotatedBibliographies.CourseContent.CourseOrganization.Cultural Differences. "'Education. Educational Guidance. Educational History. EducationalLegislation. Educational Planning.EducationalPolicy.EducationalPractice. Educational Problems. English.*Foreign Countries. International Education. Singhalese. Tamil. Teaching Methods Identifiers-Ceylon Included inthis second issue of a 3-volume series of bibliographieswith abstracts are 115 items dealing with significantmaterials published in Ceylon on various aspects of education. Titles inTamil and Singhalese are translated into English. Special attention is given to the sublect of 'educationalproblems. planning. and policy, with listings covering (1) education and the state.(2) equalization of educational opportunity. (3) language issues in education. ana(4) university education. Alongwithentriespertainingtoeducationalhistory.the bibliography places considerable emphasis on items concerning the content of educationand methods of teaching. Other sublects treated are--(1) educational commissions, committees. theory. and legislation. (2) child and youth psychology (guidance andcounseling). (3) special, vocational, technical, teacher, and adult education. and(4) examinations. Also provided are a list of the periodicals and newspapers abstracted and anauthor index. (AF) ews U.S. DEPARTMENT OF HEALTH. EDUCATION & WELFARE OFFICE OF EDUCATION THIS DOCUMENT HAS BEEN REPRODUCED EXACTLY AS RECEIVED FROM THE PERSON OR ORGANIZATION ORIGINATING IT.POINTS OF VIEW OR OPINIONS STATED DO NOT NECESSARILY REPRESENT OFFICIAL OFFICE OF EDUCATION POSITION OR POLICY. CEYLON EDUCATION ABSTRACTS January 1, 1960 to December 31, 1962. -

Open Wounds and Mounting Dangers
HUMAN OPEN WOUNDS AND RIGHTS MOUNTING DANGERS WATCH Blocking Accountability for Grave Abuses in Sri Lanka Open Wounds and Mounting Dangers Blocking Accountability for Grave Abuses in Sri Lanka Copyright © 2021 Human Rights Watch All rights reserved. Printed in the United States of America ISBN: 978-1-62313-887-5 Cover design by Rafael Jimenez Human Rights Watch defends the rights of people worldwide. We scrupulously investigate abuses, expose the facts widely, and pressure those with power to respect rights and secure justice. Human Rights Watch is an independent, international organization that works as part of a vibrant movement to uphold human dignity and advance the cause of human rights for all. Human Rights Watch is an international organization with staff in more than 40 countries, and offices in Amsterdam, Beirut, Berlin, Brussels, Chicago, Geneva, Goma, Johannesburg, London, Los Angeles, Moscow, Nairobi, New York, Paris, San Francisco, Sydney, Tokyo, Toronto, Tunis, Washington DC, and Zurich. For more information, please visit our website: http://www.hrw.org FEBRUARY 2021 ISBN: 978-1-62313-887-5 Open Wounds and Mounting Dangers Blocking Accountability for Grave Abuses in Sri Lanka Summary ......................................................................................................................... 1 Silencing Victim’s Families and Critics ......................................................................................4 Conflict-Era Violations and Failure of Accountability ................................................................ -

සාමදාන විනිශ්යකාර තනතුරු ඇතුලත් ගැසට් නිවේදනය2017-07-07(I-I)
N. B. - Parts II, III and IV (A) of the Gazette No. 2026 of 30.06.2017 were not published. YS% ,xld m%cd;dka;s%l iudcjd§ ckrcfha .eiÜ m;%h The Gazette of the Democratic Socialist Republic of Sri Lanka wxl 2"027 – 2017 cQ,s ui 07 jeks isl=rdod – 2017'07'07 No. 2,027 – FRIDAY, JULY 07, 2017 (Published by Authority) PART I : SECTION (I) – GENERAL (Separate paging is given to each language of every Part in order that it may be fi led separately) PAGE PAGE Proclamations, &c., by the President … — Government Notifi cations … … 1078 Appointments, &c., by the President … 1018 Price Control Orders … … — Appointments, &c., by the Cabinet of Ministers … — Central Bank of Sri Lanka Notices… … — Appointments, &c., by the Public Service Commission — Accounts of the Government of Sri Lanka … — Revenue and Expenditure Returns… … — Appointments, &c., by the Judicial Service Commission — Miscellaneous Departmental Notices … 1079 Other Appointments, &c. … … 1032 Notice to Mariners … … — Appointments, &c., of Registrars … — “Excise Ordinance” Notices … … — IMPORTANT NOTICE REGARDING ACCEPTANCE OF NOTICES FOR PUBLICATION IN THE WEEKLY “GAZETTE” ATTENTION is drawn to the Notifi cation appearing in the 1st week of every month, regarding the latest dates and times of acceptance of Notices for publication in the weekly Gazettes, at the end of every weekly Gazette of Democratic Socialist Republic of Sri Lanka. All notices to be published in the weekly Gazettes shall close at 12.00 noon of each Friday, two weeks before the date of publication. All Government Departments, Corporations, Boards, etc. are hereby advised that Notifi cations fi xing closing dates and times of applications in respect of Post-Vacancies, Examinations, Tender Notices and dates and times of Auction Sales, etc.