Hemidesmosomes Show Abnormal Association with the Keratin Filament Network in Junctional Forms of Epidermolysis Bullosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Vocabulari De Biologia Cel·Lular
portada biologia cel·lular 1/2/09 21:45 Página 2 VO VOCA BU LA català castellà anglès R iDE BIOLOGIA biologia cel·lular U UNIVERSITAT DE BARCELONA B portada biologia cel·lular 1/2/09 21:45 Página 3 © Comissió de Dinamització Lingüística © Serveis Lingüístics de la Facultat de Biologia de la Universitat de Barcelona de la Universitat de Barcelona Direcció: Presidenta: Conxa Planas Mercè Durfort Àrea de dinamització: Revisió conceptual: Montserrat Lleopart Mercè Durfort Àrea de terminologia: Becaris de la CDL: Àngels Egea Eduard Balbuena M. del Mar Barsó © d’aquesta edició: Serveis Lingüístics de la Universitat de Barcelona Primera edició: 1995 Segona edició revisada i ampliada: 2005 Disseny: CASS Producció: CEVAGRAF, SCCL DL: B-9744-2005 ISBN: 84-95817-08-X Interior biologia cel·lular OK 1/2/09 21:41 Página 1 OCABULARI de biologia cel·lular Presentació En el marc del procés de normalització lingüística engegat per la Universitat de Barcelona, la Comissió de Dinamització Lingüística de la Facultat de Biologia pre- senta aquesta segona edició del Vocabu- lari de biologia cel·lular, que recull la ter- minologia més habitual en l’ensenyament d’aquesta disciplina. És per això que aquesta obra s’adreça especialment als alumnes de la nostra Facultat o d’altres facultats que cursen assignatures relacio- nades amb la biologia cel·lular. Aquest vocabulari es va formar inicial- ment a partir d’un buidatge d’apunts de classe i d’obres de referència bàsiques, amb la finalitat de seleccionar els termes més utilitzats en la docència d’aquesta matèria. En aquesta segona edició s’ha ampliat considerablement el nombre d’entrades inicial i s’han afegit les equi- valències a l’anglès. -

Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D
ORIGINAL ARTICLE Plakoglobin Is Required for Effective Intermediate Filament Anchorage to Desmosomes Devrim Acehan1, Christopher Petzold1, Iwona Gumper2, David D. Sabatini2, Eliane J. Mu¨ller3, Pamela Cowin2,4 and David L. Stokes1,2,5 Desmosomes are adhesive junctions that provide mechanical coupling between cells. Plakoglobin (PG) is a major component of the intracellular plaque that serves to connect transmembrane elements to the cytoskeleton. We have used electron tomography and immunolabeling to investigate the consequences of PG knockout on the molecular architecture of the intracellular plaque in cultured keratinocytes. Although knockout keratinocytes form substantial numbers of desmosome-like junctions and have a relatively normal intercellular distribution of desmosomal cadherins, their cytoplasmic plaques are sparse and anchoring of intermediate filaments is defective. In the knockout, b-catenin appears to substitute for PG in the clustering of cadherins, but is unable to recruit normal levels of plakophilin-1 and desmoplakin to the plaque. By comparing tomograms of wild type and knockout desmosomes, we have assigned particular densities to desmoplakin and described their interaction with intermediate filaments. Desmoplakin molecules are more extended in wild type than knockout desmosomes, as if intermediate filament connections produced tension within the plaque. On the basis of our observations, we propose a particular assembly sequence, beginning with cadherin clustering within the plasma membrane, followed by recruitment of plakophilin and desmoplakin to the plaque, and ending with anchoring of intermediate filaments, which represents the key to adhesive strength. Journal of Investigative Dermatology (2008) 128, 2665–2675; doi:10.1038/jid.2008.141; published online 22 May 2008 INTRODUCTION dense plaque that is further from the membrane and that Desmosomes are large macromolecular complexes that mediates the binding of intermediate filaments. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease Aaron Buckley and Jerrold R. Turner Departments of Pathology and Medicine (Gastroenterology), Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts 02115 Correspondence: [email protected] Mucosal surfaces are lined by epithelial cells. In the intestine, the epithelium establishes a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing intrusion by luminal materials. Intestinal epithelia therefore play a central role in regulating interactions between the mucosal immune system and luminal contents, which include dietary antigens, a diverse intestinal microbiome, and pathogens. The paracellular space is sealed by the tight junction, which is maintained by a complex network of protein interactions. Tight junction dysfunction has been linked to a variety of local and systemic diseases. Two molecularly and biophysically distinct pathways across the intestinal tight junc- tion are selectively and differentially regulated by inflammatory stimuli. This review discusses the mechanisms underlying these events, their impact on disease, and the potential of using these as paradigms for development of tight junction-targeted therapeutic interventions. ucosal surfaces and the epithelial cells that adherens). The tight junction is a selectively Mline them are present at sites where tissues permeable barrier that generally represents the interface directly with the external environment rate-limiting step of paracellular transport. The or internal compartments that are contiguous adherens junction and desmosome provide es- with the external environment. Examples in- sential adhesive and mechanical properties that clude the gastrointestinal tract, the pulmonary contribute to barrier function but do not seal tree, and the genitourinary tract. -

Cytoplasmic Plaque Formation in Hemidesmosome Development Is Dependent on Soxf Transcription Factor Function
Cytoplasmic Plaque Formation in Hemidesmosome Development Is Dependent on SoxF Transcription Factor Function Shelly Oommen1, Mathias Francois2, Maiko Kawasaki1, Melanie Murrell2, Katsushige Kawasaki1, Thantrira Porntaveetus1, Sarah Ghafoor1, Neville J. Young2, Yoshimasa Okamatsu3, John McGrath4, Peter Koopman2, Paul T. Sharpe1, Atsushi Ohazama1,3* 1 Craniofacial Development and Stem Cell Biology, and Biomedical Research Centre, Dental Institute, King’s College London, London, United Kingdom, 2 Institute for Molecular Bioscience, The University of Queensland, Brisbane, Australia, 3 Department of Periodontology, Showa University Dental School, Tokyo, Japan, 4 Genetic Skin Disease Group, St John’s Institute of Dermatology, Division of Skin Sciences, King’s College London, London, United Kingdom Abstract Hemidesmosomes are composed of intricate networks of proteins, that are an essential attachment apparatus for the integrity of epithelial tissue. Disruption leads to blistering diseases such as epidermolysis bullosa. Members of the Sox gene family show dynamic and diverse expression patterns during development and mutation analyses in humans and mice provide evidence that they play a remarkable variety of roles in development and human disease. Previous studies have established that the mouse mutant ragged-opossum (Raop) expresses a dominant-negative form of the SOX18 transcription factor that interferes with the function of wild type SOX18 and of the related SOXF-subgroup proteins SOX7 and 217. Here we show that skin and oral mucosa in homozygous Raop mice display extensive detachment of epithelium from the underlying mesenchymal tissue, caused by tearing of epithelial cells just above the plasma membrane due to hemidesmosome disruption. In addition, several hemidesmosome proteins expression were found to be dysregulated in the Raop mice. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Molecular Organization of the Desmosome As Revealed by Direct Stochastic Optical Reconstruction Microscopy Sara N
© 2016. Published by The Company of Biologists Ltd | Journal of Cell Science (2016) 129, 2897-2904 doi:10.1242/jcs.185785 SHORT REPORT Molecular organization of the desmosome as revealed by direct stochastic optical reconstruction microscopy Sara N. Stahley1, Emily I. Bartle1, Claire E. Atkinson2, Andrew P. Kowalczyk1,3 and Alexa L. Mattheyses1,* ABSTRACT plakoglobin and plakophilin, and the plakin family member Desmosomes are macromolecular junctions responsible for providing desmoplakin contribute to the intracellular plaque (Fig. 1A). strong cell–cell adhesion. Because of their size and molecular Plaque ultrastructure is characterized by two electron-dense complexity, the precise ultrastructural organization of desmosomes regions: the plasma-membrane-proximal outer dense plaque and is challenging to study. Here, we used direct stochastic optical the inner dense plaque (Desai et al., 2009; Farquhar and Palade, reconstruction microscopy (dSTORM) to resolve individual plaque 1963; Stokes, 2007). The cadherin cytoplasmic tails bind to proteins pairs for inner and outer dense plaque proteins. Analysis methods in the outer dense plaque whereas the C-terminus of desmoplakin based on desmosomal mirror symmetry were developed to measure binds to intermediate filaments in the inner dense plaque. This plaque-to-plaque distances and create an integrated map. We tethers the desmosome to the intermediate filament cytoskeleton, quantified the organization of desmoglein 3, plakoglobin and establishing an integrated adhesive network (Bornslaeger et al., desmoplakin (N-terminal, rod and C-terminal domains) in primary 1996; Harmon and Green, 2013). human keratinocytes. Longer desmosome lengths correlated with Many desmosomal protein interactions have been characterized increasing plaque-to-plaque distance, suggesting that desmoplakin is by biochemical studies (Bass-Zubek and Green, 2007; Green and arranged with its long axis at an angle within the plaque. -

Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M
14 The Open Dermatology Journal, 2010, 4, 14-20 Open Access Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M. Brandner1,§, Marek Haftek*,2,§ and Carien M. Niessen3,§ 1Department of Dermatology and Venerology, University Hospital Hamburg-Eppendorf, Hamburg, Germany 2University of Lyon, EA4169 Normal and Pathological Functions of Skin Barrier, E. Herriot Hospital, Lyon, France 3Department of Dermatology, Center for Molecular Medicine, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Germany Abstract: The skin is an indispensable barrier which protects the body from the uncontrolled loss of water and solutes as well as from chemical and physical assaults and the invasion of pathogens. In recent years several studies have suggested an important role of intercellular junctions for the barrier function of the epidermis. In this review we summarize our knowledge of the impact of adherens junctions, (corneo)-desmosomes and tight junctions on barrier function of the skin. Keywords: Cadherins, catenins, claudins, cell polarity, stratum corneum, skin diseases. INTRODUCTION ADHERENS JUNCTIONS The stratifying epidermis of the skin physically separates Adherens junctions are intercellular structures that couple the organism from its environment and serves as its first line intercellular adhesion to the cytoskeleton thereby creating a of structural and functional defense against dehydration, transcellular network that coordinate the behavior of a chemical substances, physical insults and micro-organisms. population of cells. Adherens junctions are dynamic entities The living cell layers of the epidermis are crucial in the and also function as signal platforms that regulate formation and maintenance of the barrier on two different cytoskeletal dynamics and cell polarity. -
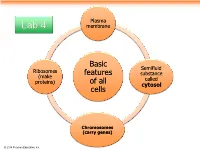
Basic Features of All Cells
Plasma Lab 4 membrane Basic Semifluid Ribosomes features substance (make called proteins) of all cytosol cells Chromosomes (carry genes) © 2014 Pearson Education, Inc. Prokaryotic cells are characterized by having . No nucleus . DNA in an unbound region called the nucleoid . No membrane-bound organelles . Cytoplasm bound by the plasma membrane © 2014 Pearson Education, Inc. Eukaryotic cells are characterized by having • DNA in a nucleus that is bounded by a membranous nuclear envelope • Membrane-bound organelles • Cytoplasm in the region between the plasma membrane and nucleus Eukaryotic cells are generally much larger than prokaryotic cells © 2014 Pearson Education, Inc. Figure 6.8a ENDOPLASMIC RETICULUM (ER) Nuclear envelope Rough ER Smooth ER Nucleolus NUCLEUS Flagellum Chromatin Centrosome Plasma membrane CYTOSKELETON: Microfilaments Intermediate filaments Microtubules Ribosomes Microvilli Golgi apparatus Peroxisome Lysosome Mitochondrion © 2014 Pearson Education, Inc. Figure 6.8b Nuclear envelope NUCLEUS Nucleolus Rough ER Chromatin Smooth ER Ribosomes Golgi Central vacuole apparatus Microfilaments CYTOSKELETON Microtubules Mitochondrion Peroxisome Plasma Chloroplast membrane Cell wall Plasmodesmata Wall of adjacent cell © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. Table 6.1 © 2014 Pearson Education, Inc. Cell Walls of Plants The cell wall is an extracellular structure that distinguishes plant cells from animal cells Plant cell walls Prokaryotes, are made -

Current Insights Into the Formation and Breakdown of Hemidesmosomes
Review TRENDS in Cell Biology Vol.16 No.7 July 2006 Current insights into the formation and breakdown of hemidesmosomes Sandy H.M. Litjens1, Jose´ M. de Pereda2 and Arnoud Sonnenberg1 1Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands 2Instituto de Biologia Molecular y Celular del Cancer, Centro de Investigacion del Cancer, University of Salamanca - CSIC, E-37007 Salamanca, Spain Hemidesmosomes are multiprotein adhesion of a6b4 [5]. Stable attachment of basal keratinocytes to the complexes that promote epithelial stromal attachment basement membrane through hemidesmosomes is of in stratified and complex epithelia. Modulation of their fundamental importance for maintaining skin integrity; function is of crucial importance in a variety of biological inherited or acquired diseases in which either of the processes, such as differentiation and migration of hemidesmosome components are affected lead to a variety keratinocytes during wound healing and carcinoma of skin blistering disorders [6–8]. invasion, in which cells become detached from the The a6b4 integrin has been associated not only with substrate and acquire a motile phenotype. Although stable adhesion, but also with cell migration [3,9,10]. In all much is known about the signaling potential of the a6b4 studies investigating keratinocyte migration or carcinoma integrin in carcinoma cells, the events that coordinate cell invasion, hemidesmosomes are disassembled, their the disassembly of hemidesmosomes during differen- components are redistributed and a6b4 is no longer tiation and wound healing remain unclear. The binding exclusively concentrated at the basal cell surface [9,10]. of a6b4 to plectin has a central role in hemidesmosome Here, we focus on recent findings on the structural assembly and it is becoming clear that disrupting this properties of hemidesmosomes and their constituents, interaction is a crucial event in hemidesmosome and the regulation of their assembly and disassembly. -

Nanoscale ARTICLE
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of ChemistryPlease do2019 not adjust margins Nanoscale ARTICLE The Impact of Tomato Fruits Containing Multi-walled Carbon Nanotube Residues on Human Intestinal Epithelial Cell Barrier Accepted 00th January 20xx Function and Intestinal Microbiome Composition a,b b b c,d c DOI: 10.1039/x0xx00000x Mohamed H. Lahiani , Sangeeta Khare , Carl E. Cerniglia , Ramiz Boy , Ilia Ivanov , and Mariya Khodakovskaya1* www.rsc.org/ a. Department of Biology, University of Arkansas at Little Rock, Little Rock, AR, 72204, USA e-mail: [email protected] b. Division of Microbiology, National Center for Toxicological Research, FDA, Jefferson, AR, 72079. c. Center for Nanophase Materials Sciences, Oak Ridge National Laboratory, Oak Ridge, TN, 37831, USA d. .Department of Textile Engineering, Çorlu Faculty of Engineering, Namık Kemal University, Çorlu-Tekirdağ 59860, TURKEY † Footnotes relating to the title and/or authors should appear here. Electronic Supplementary Information (ESI) available:Fig. S1 is Bright field microscopy of T-84 cells after exposure to CNT, Fig. S2 is shematic diagram o fNGS data analysis, Fig. S3-5 deatiled heatmap of top significantly altered genes, Table S1- 3 include list of gene classification, Fig. S6 confirmation of NGS data using Real-time PCR, Fig S7-9 PCA based on time, indivudals and doses used. See DOI: 10.1039/x0xx00000x J. Name., 2013, 00, 1-3 | 1 This journal is © The Royal Society of Chemistry 20xx Please do not adjust margins Figure S1. Bright field microscopy of T-84 cells after exposure to CNT at different doses (0.001, 0.1, and 10 µg/ml), control fruits extract (CP4), and CNT-containing fruits (CNT4) during two-time points (1- and 48-hour post-exposures).