Nutritional Ecology and the Conservation of Mona Monkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation Strategies of Wildlife Resources in the Old Oyo National Park, Nigeria
International Journal of Advanced Scientific and Technical Research Issue 4 volume 4, July-August 2014 Available online on http://www.rspublication.com/ijst/index.html ISSN 2249-9954 CONSERVATION STRATEGIES OF WILDLIFE RESOURCES IN THE OLD OYO NATIONAL PARK, NIGERIA. 1 2 3 Toyobo, Adigun E. Raheem, Wasiu M. Oyeleye, Oyewale I. 1,2&3Department of Urban and Regional Planning, Faculty of Environmental Sciences, Ladoke Akintola University of Technology, P.M.B 4000, Ogbomoso, Oyo State, Nigeria. E-mails: [email protected] [email protected] [email protected] ABSTRACT Wildlife and plants are major part of nature which goes to a large extent in providing means of livelihood for varying categories of people in the world. Human beings are however engaged in over exploitation of this segment of the ecosystem, so much that many species are becoming endangered. It is against this background that this study evaluates the conservation strategies of wildlife resources in Nigerian national parks with particular reference to the old Oyo National Park. Data used for the study were primary data obtained through random sampling of 60 respondents in Ikoyi-Ile, an adjoining settlement, richer in the fauna population of the park. Data collected were analyzed using descriptive statistic and chi square statistics. The result shows considerable degree of effectiveness of the strategies employed by the authority of the park, as majority of the respondents are aware of the status of the park as a protected area. The paper therefore concludes by emphasizing the need to brace up in the various conservation programmes of the park. -

Lemur News 7 (2002).Pdf
Lemur News Vol. 7, 2002 Page 1 Conservation International’s President EDITORIAL Awarded Brazil’s Highest Honor In recognition of his years of conservation work in Brazil, CI President Russell Mittermeier was awarded the National Are you in favor of conservation? Do you know how conser- Order of the Southern Cross by the Brazilian government. vation is viewed by the academic world? I raise these ques- Dr. Mittermeier received the award on August 29, 2001 at tions because they are central to current issues facing pri- the Brazilian Ambassador's residence in Washington, DC. matology in general and prosimians specifically. The National Order of the Southern Cross was created in The Duke University Primate Center is in danger of being 1922 to recognize the merits of individuals who have helped closed because it is associated with conservation. An inter- to strengthen Brazil's relations with the international com- nal university review in 2001 stated that the Center was too munity. The award is the highest given to a foreign national focused on conservation and not enough on research. The re- for service in Brazil. viewers were all researchers from the "hard" sciences, but For the past three decades, Mittermeier has been a leader in they perceived conservation to be a negative. The Duke ad- promoting biodiversity conservation in Brazil and has con- ministration had similar views and wanted more emphasis ducted numerous studies on primates and other fauna in the on research and less on conservation. The new Director has country. During his time with the World Wildlife Fund three years to make that happen. -

Assessment of Population Density and Structure of Primates in Pandam Wildlife Park, Plateau State, Nigeria
Sustainability, Agri, Food and Environmental Research, (ISSN: 0719-3726) , 6(2), 2018: 18-35 18 http://dx.doi.org/10.7770/safer-V6N2-art1503 ASSESSMENT OF POPULATION DENSITY AND STRUCTURE OF PRIMATES IN PANDAM WILDLIFE PARK, PLATEAU STATE, NIGERIA DENSIDAD POBLACIONAL Y ESTRUCTURA DE PRIMATES DIURNOS EN EL PARQUE DE VIDA SILVESTRE EN PANDAM, ESTADO DE PLATEAU, NIGERIA. Gabriel Ortyom Yager*, James Oshita Bukie and Avalumun Emmanuel Kaa, Department of Wildlife and Range Management, University of Agriculture, Makurdi, Benue State, Nigeria. *Correspondent author email & Phone: [email protected]; 08150609846 ABSTRACT A survey of diurnal primate species in Pandam Wildlife Park, Nigeria was conducted to determine its population density and structure. Eight transect lines (2.0km length, 0.02km width) at interval of 1.0km were located as representative samples in the Park within the Three-range stratum (riparian forest, savannah woodland and, swampy area) based on proportional to size of the strata in providing information on the primates’ species present in the Park which include Cercopithecus mona, Erythrocebus patas, Papio anubis and, Chlorocebus tantalus. Direct method of animal sighting was employed. Data was collected and analyzed using descriptive statistics, ANOVA and diversity indices. The results showed that savannah woodland strata had more number of individual species encountered (132) and the lowest was the swampy area. Also the savannah woodland had the highest species diversity and richness while the riparian forest strata had the highest number of species evenness. More so, Cercopithecus tantalus was widespread throughout the Park among other primates and Cercopithecus mona is most likely to decline even more rapidly than others since they inhabit the very tall trees. -

Comments on the Ornithology of Nigeria, Including Amendments to the National List
Robert J. Dowsett 154 Bull. B.O.C. 2015 135(2) Comments on the ornithology of Nigeria, including amendments to the national list by Robert J. Dowsett Received 16 December 2014 Summary.—This paper reviews the distribution of birds in Nigeria that were not treated in detail in the most recent national avifauna (Elgood et al. 1994). It clarifies certain range limits, and recommends the addition to the Nigerian list of four species (African Piculet Verreauxia africana, White-tailed Lark Mirafra albicauda, Western Black-headed Batis Batis erlangeri and Velvet-mantled Drongo Dicrurus modestus) and the deletion (in the absence of satisfactory documentation) of six others (Olive Ibis Bostrychia olivacea, Lesser Short-toed Lark Calandrella rufescens, Richard’s Pipit Anthus richardi, Little Grey Flycatcher Muscicapa epulata, Ussher’s Flycatcher M. ussheri and Rufous-winged Illadopsis Illadopsis rufescens). Recent research in West Africa has demonstrated the need to clarify the distributions of several bird species in Nigeria. I have re-examined much of the literature relating to the country, analysed the (largely unpublished) collection made by Boyd Alexander there in 1904–05 (in the Natural History Museum, Tring; NHMUK), and have reviewed the data available in the light of our own field work in Ghana (Dowsett-Lemaire & Dowsett 2014), Togo (Dowsett-Lemaire & Dowsett 2011a) and neighbouring Benin (Dowsett & Dowsett- Lemaire 2011, Dowsett-Lemaire & Dowsett 2009, 2010, 2011b). The northern or southern localities of species with limited ranges in Nigeria were not always detailed by Elgood et al. (1994), although such information is essential for understanding distribution patterns and future changes. For many Guineo-Congolian forest species their northern limit in West Africa lies on the escarpment of the Jos Plateau, especially Nindam Forest Reserve, Kagoro. -

Lighting up the Arena
The African e-Journals Project has digitized full text of articles of eleven social science and humanities journals. This item is from the digital archive maintained by Michigan State University Library. Find more at: http://digital.lib.msu.edu/projects/africanjournals/ Available through a partnership with Scroll down to read the article. Duro Oni examines the role of lighting in the contemporary Nigerian theater with emphasis on theatrical activities in Arena Lagos. .is Lighting? A good itartin.g DOint will involve reluming to •rancis Reid's2 observation that "Stage ighting is not an exact science: it is science in the service, of performing arts. Rules are very few it indeed there are any." Lighting is a combination of science and art; science being the understgnding of trie technology in the proguction of righting instruments and equipment and art being the creative use of such instruments and equipment. For. lighting is as much a oqrt of the artistic process as any of the other aspects of the theater, helping to create the necessary atmosphere and mood for <GLENDORA REVltVfxAlriain Quarterly on the ArtsxVOI3@No2> <107> 0 dramatiC presentation. In defining stage lighting, Reid3 summarized the main aims of lighting for theatrical productions thus: Stage lighting is a fluid, selective, atmospheric, sculptural illumination appropriate to the style of a particular production. Reid's definition prescribes the primary functions of stage lighting - and accepted by most writers on the subject — as illumination, selectivity, fluidity, atmosphere, mood, dimensionality and creating effects. It was not until recently that the functions of stage lighting were further elaborated upon. -

S/N Company Name Address Licence Number
CLASS LICENCE REGISTER REPAIRS AND MAINTENANCE S/N COMPANY NAME ADDRESS LICENCE NUMBER 1 Telecare Wireless Ltd 13, Amodu Ojikutu Street, V/I, Lagos CL/R&M/001/07 2 Lintech TelecommunicationsSolution Nig Ltd 18am Temple Road, Ikoyi PMB 40014, Falomo, Lagos CL/R&M/002/07 3 Juvs Acme Network Ltd Plot 53, Agege Motor Road, Ladipo Opp FRCN - GRA) Ikeja Lagos CL/R&M/003/07 4 Stepping Forth TechnologyConcepts Ltd Shop A5, Zuma Mall, 20 Ndola Crescent, Wuse Zone5, Abuja CL/R&M/004/07 5 Or - be -com Nig Ltd 21/23, Flat 7, Block 7, Taslim Elias Close, V/I, Lagos CL/R&M/005/07 6 Phoneport Services Centre Ltd No 25, Kampala Street, Wuse II, Abuja CL/R&M/006/07 7 Camtec Nig Ltd 201B, Corporation Drive Dolphin, Estate Ikoyi, Lagos CL/R&M/007/07 8 Telecom Eng Tools Ltd Suite 18, Gwamna Complex, N3, Kachia Road, Kaduna CL/R&M/008/07 9 Wireless Point Communications& Technologies Ltd No 51 Constitution Avenue, Gaduwa Housing EstateAbuja CL/R&M/009/07 10 Qrec Nig Ltd No 9, New Era Road, Iyana - Ipaja, Lagos CL/R&M/010/07 11 Barotech Nig Ltd 20, Mbonu Street, D - Line Port Harcourt Rivers State CL/R&M/001/08 12 BT Technologies Ltd 6th Floor Bookshop House 50 -52, Broad Street, Lagos CL/R&M/002/08 13 Spar Aerospace Nig Ltd 3A Aja Nwachukwu Close, Ikoyi Lagos CL/R&M/003/08 14 Smartway Technologies Ltd 155, Olojo Drive, Ojo Lagos CL/R&M/004/08 15 Steve Power - Mart Ltd 11, Olatubosun Street, Sonibare Estate, MarylandLagos CL/R&M/005/08 16 Dagbs Nig Ltd Suite 101, Dolphin Plaza Dolphin Estate, Ikoyi, Lagos CL/R&M/006/08 17 Chuks Telecoms GlobalResources Ltd 24 Kirikiri Rd, 2nd Floor, Flat2, Olodi Apapa, Lagos CL/R&M/007/08 18 Peak Mark Procurement Ltd 28, Osinowo Street, Gbagada, Lagos CL/R&M/008/08 19 Protean Global Nig Ltd 234A, Muki Okunola Street, V/I, Lagos CL/R&M/009/08 1 CLASS LICENCE REGISTER REPAIRS AND MAINTENANCE 20 Western Development Co. -

ISSN: 2149-6528 2016 Vol. 1, Issue.1 SUSTAINABLE WILDLIFE
Journal of Tourism and Management Research 101 ISSN:2149-6528 Journal of Tourism and Management Research ISSN: 2149-6528 2016 Vol. 1, Issue.1 SUSTAINABLE WILDLIFE CONSERVATION AT OKOMU NATIONAL PARK, NIGERIA Abstract The purpose of this study is to investigate the various sustainable preservation measures at Okomu National Park, Benin, Nigeria, identify the various challenges of the Park and suggest the way forward. Ethnography was adopted where research methods like key-informant interview, focus-group discussion, field observation, and electronic documentation were used to elicit data from the field. Relevant documentary sources were not left out either. Data gathered were analysed using descriptive and narrative analytical methods. This revealed a range of management measures that were grouped into two main categories (i.e. Educational measures and Technical measures). Major challenges like poaching and community violence were identified, with the major causes arising from host communities. At the end the study made a useful contribution to the sustainable management of parks for ecotourism development, by introducing the HoPSuP Model as a management practice to encourage a healthy host-park relationship in Nigeria and some other developing nations. Keywords: sustainability, wildlife, conservation, national park, challenges, HoPSuP model. _________________________________ Elochukwu A. Nwankwo, PhD. Department of Archaeology and Tourism / University of Nigeria Nsukka. Email: [email protected] Aishat Halilu, M.A. Department of Archaeology and Tourism / University of Nigeria, Nsukka. Email: [email protected] Original Scientific Paper Nwankwo, E.A and Halilu, A. Vol.1/No.1/2016/101-118. DOI:10.26465/ojtmr.2016132258 Journal of Tourism and Management Research 102 1. -

Habitat Are of Special Scientific, Educative And
Ethiopian Journal of Environmental Studies and Management Vol. 4 No.3 2011 ASSESSMENT OF THE LARGE MAMMALS OF ARAKHUAN RANGE, OKOMU NATIONAL PARK, NIGERIA Akinsorotan, O. A., *Ogunjemite, B. G. and Afolayan, T. A. DOI:http://dx.doi.org/10.4314/ejesm.v4i3.4 Received September 26 th 2011; accepted October 5 th 2011 Abstract An assessment of the large mammals of Arakhuan Range, Okomu National Park, Edo State, Nigeria, was carried out using land transect methods. A total of 12 large mammalian species were observed (eight directly and four by their signs). These are made up of four primate species; Red-capped mangabey (Cercocebus torquatus), Mona monkey (Cercopithecus mona), White-throated monkey (C. erythrogaster pocoki), and Putty-nosed monkey (C. nictitans ludio), three species of antelope: Maxwell duiker (Cephalophus maxwelli), Yellow-backed duiker (Cephalophus silvicultor), and Red-flanked duiker (Cephalophus rufilatus), and a species of mangoose (Herpestes sp). Those observed through their activities were forest elephants (Loxodonta africana cylotis), forest buffalo (Syncerus caffer nanus), civet cat (Viverra civetta) and the red river hog (Patamochoerus porcus). Altogether, 145 sightings of animals were recorded during the study period. Mona monkeys were most commonly sighted (sighted 37 times, producing 0.22 sighting/km). The Red-flanked duiker was the most abundant with an estimated density of 36.66±7.68 km -2 and population of 1970.93±412.93 individuals. The yellow-backed duiker was very rare in the range as it was sighted three times with an estimated population of 9 ± 2 individuals. There is very strong correlation between sightings in the morning and that of evening (r = 0.94, P < 0.05). -

UNIVERSITY of CALGARY Determinants of Group Size and Composition for Colobus Vellerosus at Boabeng-Fiema, Ghana
UNIVERSITY OF CALGARY Determinants of Group Size and Composition for Colobus vellerosus at Boabeng-Fiema, Ghana: Ecological versus Social Factors by Julie A. Teichroeb A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF ANTHROPOLOGY CALGARY, ALBERTA MARCH, 2009 © Julie A. Teichroeb 2009 UNIVERSITY OF CALGARY FACULTY OF GRADUATE STUDIES The undersigned certify that they have read, and recommend to the Faculty of Graduate Studies for acceptance, a thesis titled “Determinants of Group Size and Composition for Colobus vellerosus at Boabeng-Fiema, Ghana: Ecological versus Social Factors” submitted by Julie A. Teichroeb in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ___________________________________ Supervisor, Dr. Pascale Sicotte, Department of Anthropology ___________________________________ Dr. Linda Fedigan, Department of Anthropology ___________________________________ Dr. Robert Longair, Department of Biological Sciences ___________________________________ Dr. Warren Wilson, Department of Archaeology ___________________________________ External Examiner, Dr. Rasanayagam Rudran, Scientist Emeritus, Smithsonian Institution _____________ Date ii ABSTRACT Intense within-group food competition is not thought to influence social organization for folivorous primates. Thus, they are expected by the socioecological model to live in large groups, exploiting benefits such as predation avoidance. However, many folivores form small groups, well below the threshold to avoid within-group scramble competition for food (the “folivore paradox”, Janson & Goldsmith, 1995). Social factors, rather than ecological factors, are thought to determine social organization for folivores, though this has rarely been systematically tested. In this study, the relative contribution of ecological versus social factors on the group size and composition of ursine colobus monkeys (Colobus vellerosus ) at the Boabeng-Fiema Monkey Sanctuary, Ghana was explored. -
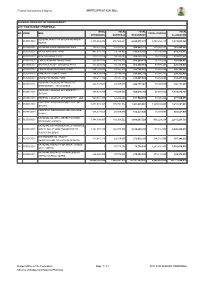
Appropriation Bill
Federal Government of Nigeria APPROPRIATION BILL FEDERAL MINISTRY OF ENVIRONMENT 2017 FGN BUDGET PROPOSAL TOTAL TOTAL TOTAL TOTAL NO CODE MDA TOTAL CAPITAL PERSONNEL OVERHEAD RECURRENT ALLOCATION FEDERAL MINISTRY OF ENVIRONMENT - 1. 0535001001 2,173,269,254 672,562,421 2,845,831,675 4,567,262,147 7,413,093,823 HQTRS 2. 0535002001 NATIONAL PARK HEADQUARTERS 193,634,708 54,447,671 248,082,379 514,004,581 762,086,960 3. 0535003001 KAINJI NATIONAL PARK 482,677,775 53,198,837 535,876,612 82,500,000 618,376,613 4. 0535004001 OYO NATIONAL PARK 389,743,876 50,590,121 440,333,997 55,483,225 495,817,222 5. 0535005001 CHAD BASIN NATIONAL PARK 228,681,496 40,539,137 269,220,633 56,700,000 325,920,633 6. 0535006001 GASHAKA GUMTI NATIONAL PARK 312,443,632 50,105,537 362,549,169 57,970,463 420,519,633 7. 0535007001 CROSS RIVER NATIONAL PARK 482,142,617 50,563,970 532,706,587 93,650,000 626,356,587 8. 0535008001 KAMUKU NATIONAL PARK 194,859,429 38,796,289 233,655,718 60,666,775 294,322,493 9. 0535009001 OKUMU NATIONAL PARK 180,214,108 33,847,716 214,061,824 40,350,000 254,411,824 FEDERAL COLLEGE OF WILDLIFE 10. 0535010001 436,737,061 60,000,000 496,737,061 50,000,000 546,737,061 MANAGEMENT - NEW BUSSA FEDERAL COLLEGE OF FORESTRY - 11. 0535011001 889,578,759 70,000,000 959,578,759 60,000,000 1,019,578,759 IBADAN 12. -

Population Composition and Density of Mona Monkey in Lekki Concervation Centre, Lekki, Lagos, Nigeria
Olaleru et al., 2020 Journal of Research in Forestry, Wildlife & Environment Vol. 12(3) September, 2020 E-mail: [email protected]; [email protected] http://www.ajol.info/index.php/jrfwe This work is licensed under a jfewr ©2020 - jfewr Publications 259 Creative Commons Attribution 4.0 License ISBN: 2141 – 1778 Olaleru et al., 2020 POPULATION COMPOSITION AND DENSITY OF MONA MONKEY IN LEKKI CONCERVATION CENTRE, LEKKI, LAGOS, NIGERIA *1, 2Olaleru, F., 1Omotosho, O. O. and 1, 2Omoregie, Q. O. 1Department of Zoology, Faculty of Science, University of Lagos, Lagos State, Nigeria. 2Centre for Biodiversity Conservation and Ecosystem Management, University of Lagos, Lagos State, Nigeria. * Corresponding Author: [email protected]; + 234 807 780 0748 ABSTRACT The mona monkey (Cercopitecus mona) is the only non-human primate in Lekki Conservation Centre (LCC), a 78 hectares Strict Nature Reserve located in a peri-urban part of Lagos, Nigeria. This study aimed to of population composition and density of mona monkeys in LCC. Total count method using woods walk ways and perimeter road as line transects was used for the enumeration. The censuses were conducted for 27 days in October, November, and December, 2018. Counts were carried out between 06:30 and 10:30 hours for 22 days, and between 16:30 and 19:00 hours for five days. Monkeys were enumerated by counting all sighted individuals on both sides of the walk ways, and other study points. Data was subjected to analysis of variance to compare the monthly means, and statistically significant means (P < 0.05) were separated using Tukey post hoc test. -

Evolutionary Stasis of the Pseudoautosomal Boundary In
Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates Rylan Shearn, Alison E Wright, Sylvain Mousset, Corinne Régis, Simon Penel, Jean-François Lemaître, Guillaume Douay, Brigitte Crouau-Roy, Emilie Lecompte, Gabriel Ab Marais To cite this version: Rylan Shearn, Alison E Wright, Sylvain Mousset, Corinne Régis, Simon Penel, et al.. Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates. eLife, eLife Sciences Publication, 2020, 9, 10.7554/eLife.63650. hal-03064964 HAL Id: hal-03064964 https://hal.archives-ouvertes.fr/hal-03064964 Submitted on 14 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. SHORT REPORT Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates Rylan Shearn1, Alison E Wright2, Sylvain Mousset1,3, Corinne Re´ gis1, Simon Penel1, Jean-Franc¸ois Lemaitre1, Guillaume Douay4, Brigitte Crouau-Roy5, Emilie Lecompte5, Gabriel AB Marais1,6* 1Laboratoire Biome´trie et Biologie Evolutive, CNRS / Univ. Lyon 1, Villeurbanne, France; 2Department of Animal and Plant Sciences, University of Sheffield, Sheffield, United Kingdom; 3Faculty of Mathematics, University of Vienna, Vienna, Austria; 4Zoo de Lyon, Lyon, France; 5Laboratoire Evolution et Diversite´ Biologique, CNRS / Univ. Toulouse, Toulouse, France; 6LEAF-Linking Landscape, Environment, Agriculture and Food Dept, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal Abstract Sex chromosomes are typically comprised of a non-recombining region and a recombining pseudoautosomal region.