Detailed Species Accounts from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dams and Development in China
BRYAN TILT DAMS AND The Moral Economy DEVELOPMENT of Water and Power IN CHINA DAMS AND DEVELOPMENT CHINA IN CONTEMPORARY ASIA IN THE WORLD CONTEMPORARY ASIA IN THE WORLD DAVID C. KANG AND VICTOR D. CHA, EDITORS This series aims to address a gap in the public-policy and scholarly discussion of Asia. It seeks to promote books and studies that are on the cutting edge of their respective disciplines or in the promotion of multidisciplinary or interdisciplinary research but that are also accessible to a wider readership. The editors seek to showcase the best scholarly and public-policy arguments on Asia from any field, including politics, his- tory, economics, and cultural studies. Beyond the Final Score: The Politics of Sport in Asia, Victor D. Cha, 2008 The Power of the Internet in China: Citizen Activism Online, Guobin Yang, 2009 China and India: Prospects for Peace, Jonathan Holslag, 2010 India, Pakistan, and the Bomb: Debating Nuclear Stability in South Asia, Šumit Ganguly and S. Paul Kapur, 2010 Living with the Dragon: How the American Public Views the Rise of China, Benjamin I. Page and Tao Xie, 2010 East Asia Before the West: Five Centuries of Trade and Tribute, David C. Kang, 2010 Harmony and War: Confucian Culture and Chinese Power Politics, Yuan-Kang Wang, 2011 Strong Society, Smart State: The Rise of Public Opinion in China’s Japan Policy, James Reilly, 2012 Asia’s Space Race: National Motivations, Regional Rivalries, and International Risks, James Clay Moltz, 2012 Never Forget National Humiliation: Historical Memory in Chinese Politics and Foreign Relations, Zheng Wang, 2012 Green Innovation in China: China’s Wind Power Industry and the Global Transition to a Low-Carbon Economy, Joanna I. -

A Study of Food and Feeding Habits of Blue Peafowl, Pavo Cristatus Linnaeus, 1758 in District Kurukshetra, Haryana (India)
International Journal of Research Studies in Biosciences (IJRSB) Volume 2, Issue 6, July 2014, PP 11-16 ISSN 2349-0357 (Print) & ISSN 2349-0365 (Online) www.arcjournals.org A Study of Food and Feeding Habits of Blue Peafowl, Pavo Cristatus Linnaeus, 1758 in District Kurukshetra, Haryana (India) Girish Chopra, Tarsem Kumar Department of Zoology, Kurukshetra University, Kurukshetra-136119 (INDIA) [email protected] Summary: Present study was conducted to determine the food and feeding habits of blue peafowl in three study sites, namely, Saraswati plantation wildlife sanctuary (SPWS), Bir Sonti Reserve Forest (BSRF), and Jhrouli Kalan village (JKAL). Point count method (Blondel et al., 1981) was followed during periodic fortnightly visits to all the three selected study sites. The peafowls were observed to feed on flowers, fruits, leaves of 11, 8 and 8 plant species respectively. These were sighted to feed on Brassica compestris (flowers, leaves), Trifolium alexandarium (flowers, leaves), Triticum aestivum (flowers, leaves, fruits), Oryza sativa (flowers, leaves, fruits), Chenopodium album (flowers, leaves, fruits), Parthenium histerophoresus (flowers, leaves), Pisum sativum (flowers, leaves, fruits), Cicer arientum (flowers, leaves, fruits), Pyrus pyrifolia (flowers, fruits), Ficus benghalensis (flowers, fruits), Ficus rumphii (flowers, fruits). They were also observed feeding on insects in all three study sites and on remains of the snake bodies at the BSRF and JKAL study site. The findings revealed that the Indian peafowl, on one hand, functions as a predator of agricultural pests but, on the other hand, is itself a pest on agricultural crops. Keywords: Blue peafowl, Food, Feeding Habits, Herbs, Shrubs, Trees. 1. INTRODUCTION Birds are warm-blooded, bipedal, oviparous vertebrates characterized by bony beak, pneumatic bones, feathers and wings. -

A Study of the Composition and Dissolution of Jianshui Purple Pottery in Yunnan, China
crystals Article A Study of the Composition and Dissolution of Jianshui Purple Pottery in Yunnan, China Chang Liu 1,*, Heng Xie 2, Lei Nie 3, Hong Wang 4 and Yuanyuan He 3 1 College of Art and Design, Jilin Jianzhu University, Changchun 130021, China 2 Jianshui Purple Pottery Research Association, Jianshui 654300, China; [email protected] 3 College of Construction Engineering, Jilin University, Xi Min Zhu Street, Changchun 130026, China; [email protected] (L.N.); [email protected] (Y.H.) 4 College of Civil Engineering, Guizhou University, Guiyang 550025, China; [email protected] * Correspondence: [email protected] Abstract: Pottery is a gem in the history of human civilization and a crystallization of human wisdom. Yunnan Jianshui purple pottery is one of the four famous types of pottery in China, with a long history and superb craftsmanship. Used as tableware, research on the composition and element dissolution of pottery is extremely significant for production and health. This paper takes Jianshui purple pottery as its research object, samples its raw ores and finished products, and conducts X-ray fluorescence, scanning electron microscopy, inductively coupled plasma mass spectrometry experiments, and dissolution tests. The chemical composition, microstructure, and trace element concentrations of pottery before and after firing were measured. Results show that the dissolution of purple pottery under various use scenarios is low and meets health requirements. Combined with the characteristics of purple pottery, the composition changes and the mechanism of change Citation: Liu, C.; Xie, H.; Nie, L.; before and after firing are discussed, which can be used as the theoretical basis for improving pottery Wang, H.; He, Y. -

Militarized Conflicts in Northern Shan State
A Return to War: Militarized Conflicts in Northern Shan State ASIA PAPER May 2018 EUROPEAN UNION A Return to War: Militarized Conflicts in Northern Shan State © Institute for Security and Development Policy V. Finnbodavägen 2, Stockholm-Nacka, Sweden www.isdp.eu “A Return to War: Militarized Conflicts in Northern Shan State” is an Asia Paper published by the published by the Institute for Security and Development Policy. The Asia Paper Series is the Occasional Paper series of the Institute’s Asia Program, and addresses topical and timely subjects. The Institute is based in Stockholm, Sweden, and cooperates closely with research centers worldwide. The Institute serves a large and diverse community of analysts, scholars, policy-watchers, business leaders, and journalists. It is at the forefront of research on issues of conflict, security, and development. Through its applied research, publications, research cooperation, public lectures, and seminars, it functions as a focal point for academic, policy, and public discussion. This publication has been produced with funding by the European Union. The content of this publication does not reflect the official opinion of the European Union. Responsibility for the information and views expressed in the paper lies entirely with the authors. No third-party textual or artistic material is included in the publication without the copyright holder’s prior consent to further dissemination by other third parties. Reproduction is authorised provided the source is acknowledged. © European Union and ISDP, 2018 Printed in Lithuania ISBN: 978-91-88551-11-5 Cover photo: Patrick Brown patrickbrownphoto.com Distributed in Europe by: Institute for Security and Development Policy Västra Finnbodavägen 2, 131 30 Stockholm-Nacka, Sweden Tel. -

Report on Domestic Animal Genetic Resources in China
Country Report for the Preparation of the First Report on the State of the World’s Animal Genetic Resources Report on Domestic Animal Genetic Resources in China June 2003 Beijing CONTENTS Executive Summary Biological diversity is the basis for the existence and development of human society and has aroused the increasing great attention of international society. In June 1992, more than 150 countries including China had jointly signed the "Pact of Biological Diversity". Domestic animal genetic resources are an important component of biological diversity, precious resources formed through long-term evolution, and also the closest and most direct part of relation with human beings. Therefore, in order to realize a sustainable, stable and high-efficient animal production, it is of great significance to meet even higher demand for animal and poultry product varieties and quality by human society, strengthen conservation, and effective, rational and sustainable utilization of animal and poultry genetic resources. The "Report on Domestic Animal Genetic Resources in China" (hereinafter referred to as the "Report") was compiled in accordance with the requirements of the "World Status of Animal Genetic Resource " compiled by the FAO. The Ministry of Agriculture" (MOA) has attached great importance to the compilation of the Report, organized nearly 20 experts from administrative, technical extension, research institutes and universities to participate in the compilation team. In 1999, the first meeting of the compilation staff members had been held in the National Animal Husbandry and Veterinary Service, discussed on the compilation outline and division of labor in the Report compilation, and smoothly fulfilled the tasks to each of the compilers. -

Diversity of a Large Collection of Natural Populations of Mango (Mangifera Indica Linn.) Revealed by Agro-Morphological and Quality Traits
diversity Article Diversity of a Large Collection of Natural Populations of Mango (Mangifera indica Linn.) Revealed by Agro-Morphological and Quality Traits Cuixian Zhang y, Dehong Xie y, Tianqi Bai, Xinping Luo, Faming Zhang, Zhangguang Ni * and Yufu Chen * Institute of Tropical and Subtropical Cash Crops, Yunnan Academy of Agricultural Sciences, Baoshan 678000, China; [email protected] (C.Z.); [email protected] (D.X.); [email protected] (T.B.); [email protected] (X.L.); [email protected] (F.Z.) * Correspondence: [email protected] (Z.N.); [email protected] or [email protected] (Y.C.) These authors contributed equally to this work. y Received: 11 December 2019; Accepted: 3 January 2020; Published: 11 January 2020 Abstract: Collection, characterization and utilization of genetic resources are crucial for developing varieties to meet current and future needs. Although mango is an economically important fruit tree, its genetic resources are still undocumented and are threatened in their natural habits. In this study, the variability of 452 mango accessions from three regions in China (Nujiang, Lancang river and Honghe) was assessed using 41 descriptors including qualitative and quantitative traits, with the aim to identify mango accessions with excellent agronomic and quality traits. To this end, descriptive and multivariate analyses were performed. Based on Shannon–Weaver diversity index, qualitative traits including pericarp color, fruit aroma, flesh color, and fruit flavor recorded the highest variability in the germplasm. Fruit related traits including pulp weight, peel weight, and fruit weight were the most diverse traits in the germplasm with a high coefficient of variation (CV > 40%). Significant differences (MANOVA test, p < 0.000) were observed among the three regions for most of the quantitative traits. -

(Pavo Cristatus): People Perception at Annur and Avinasi Areas of Tamil Nadu, India
Journal of Zoological Research Volume 3, Issue 1, 2019, PP 6-12 ISSN 2637-5575 Conflict with Blue Peafowl (Pavo cristatus): People Perception at Annur and Avinasi areas of Tamil Nadu, India Veeramani.A1*, Dalson Mani.J2 and Mohanakrishnan.H3 1 Government Arts College (Autonomous), Kumbakonam, Tamil Nadu, India 2 Biologist, Mudumalai Tiger Reserve, The Nilgiris, Tamil Nadu, India 3 Government Arts College, Udhagamandalam, Tamil Nadu, India *Corresponding Author: Veeramani Arunachalam, Assistant Professor of Zoology, Government Arts College (Autonomous), Kumbakonam – 612 002, Tamil Nadu, India. Email: [email protected]. ABSTRACT The present investigation is indent to study on Blue Peafowl Pavo cristatus in Annur and Avinasi areas of Tamil Nadu. Abundance and problems of peafowls in selected cultivated fields were collected using questionnaire survey method. Interviews were conducted and discussion was made with the local peoples regarding the details of peafowl. The opinion on the local cultivators are the high abundance of peafowls in the study areas may be due to the availability of sufficient food plants, insects, roosting trees and a good ground cover for breeding and protection. The cultivators opined that the peafowl feeds on a wide range of crops such as beans, chilly, capsicum, tomato, maize. Only very few incidents of poisoning happened in the recent past, otherwise the peafowls are the pet animal of the people living around. Government and non- governmental agencies should keep a vigil against poaching and poisoning of the peafowls and make aware of the people about the importance of protecting our National Bird. Keywords: Peafowl, Questionnaire Survey, Roosting, Food Preferences, Conservation. -

A Molecular Phylogeny of the Peacock-Pheasants (Galliformes: Polyplectron Spp.) Indicates Loss and Reduction of Ornamental Traits and Display Behaviours
Biological Journal of the Linnean Society (2001), 73: 187–198. With 3 figures doi:10.1006/bijl.2001.0536, available online at http://www.idealibrary.com on A molecular phylogeny of the peacock-pheasants (Galliformes: Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours REBECCA T. KIMBALL1,2∗, EDWARD L. BRAUN1,3, J. DAVID LIGON1, VITTORIO LUCCHINI4 and ETTORE RANDI4 1Department of Biology, University of New Mexico, Albuquerque, NM 87131, USA 2Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, OH 43210, USA 3Department of Plant Biology, Ohio State University, Columbus, OH 43210, USA 4Istituto Nazionale per la Fauna Selvatica, via Ca` Fornacetta 9, 40064 Ozzano dell’Emilia (BO), Italy Received 4 September 2000; accepted for publication 3 March 2001 The South-east Asian pheasant genus Polyplectron is comprised of six or seven species which are characterized by ocelli (ornamental eye-spots) in all but one species, though the sizes and distribution of ocelli vary among species. All Polyplectron species have lateral displays, but species with ocelli also display frontally to females, with feathers held erect and spread to clearly display the ocelli. The two least ornamented Polyplectron species, one of which completely lacks ocelli, have been considered the primitive members of the genus, implying that ocelli are derived. We examined this hypothesis phylogenetically using complete mitochondrial cytochrome b and control region sequences, as well as sequences from intron G in the nuclear ovomucoid gene, and found that the two least ornamented species are in fact the most recently evolved. Thus, the absence and reduction of ocelli and other ornamental traits in Polyplectronare recent losses. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -
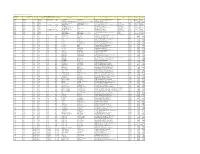
Colgate Palmolive List of Mills As of June 2018 (H1 2018) Direct
Colgate Palmolive List of Mills as of June 2018 (H1 2018) Direct Supplier Second Refiner First Refinery/Aggregator Information Load Port/ Refinery/Aggregator Address Province/ Direct Supplier Supplier Parent Company Refinery/Aggregator Name Mill Company Name Mill Name Country Latitude Longitude Location Location State AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora Agroaceite Extractora Agroaceite Finca Pensilvania Aldea Los Encuentros, Coatepeque Quetzaltenango. Coatepeque Guatemala 14°33'19.1"N 92°00'20.3"W AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora del Atlantico Guatemala Extractora del Atlantico Extractora del Atlantico km276.5, carretera al Atlantico,Aldea Champona, Morales, izabal Izabal Guatemala 15°35'29.70"N 88°32'40.70"O AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora La Francia Extractora La Francia km. 243, carretera al Atlantico,Aldea Buena Vista, Morales, izabal Izabal Guatemala 15°28'48.42"N 88°48'6.45" O Oleofinos Oleofinos Mexico Pasternak - - ASOCIACION AGROINDUSTRIAL DE PALMICULTORES DE SABA C.V.Asociacion (ASAPALSA) Agroindustrial de Palmicutores de Saba (ASAPALSA) ALDEA DE ORICA, SABA, COLON Colon HONDURAS 15.54505 -86.180154 Oleofinos Oleofinos Mexico Pasternak - - Cooperativa Agroindustrial de Productores de Palma AceiteraCoopeagropal R.L. (Coopeagropal El Robel R.L.) EL ROBLE, LAUREL, CORREDORES, PUNTARENAS, COSTA RICA Puntarenas Costa Rica 8.4358333 -82.94469444 Oleofinos Oleofinos Mexico Pasternak - - CORPORACIÓN -

Workshop Proceedings EXPERIENCES and POTENTIAL for COMMUNITY FOREST MANAGEMENT in VIETNAM
Page 1 of 2 Workshop Proceedings EXPERIENCES AND POTENTIAL FOR COMMUNITY FOREST MANAGEMENT IN VIETNAM HANOI, JUNE 1-2, 2000 TABLE OF CONTENTS iii Acronyms iv Glossary v Welcoming Speech vii Introduction viii Executive Summary Reports 1 "Current trends of community forestry in Vietnam." Nguyen Hong Quan and To Dinh Mai 6 "The CFM strategy of the Social Forestry Development Project (SFDP)." Nguyen Tuong Van and Ulrich Apel 10 "Linking Government & local forest management: A new approach to CFM being tested in Yen Bai." Edwin Shank 13 "Designing and implementing participatory forest protection & development regulations at the village level (Son La Province)." Nguyen Van Tuan CFM Case Study Summaries 18 "Muong Lum Commune, Yen Chau District, Son La Province (Thai & H’mong minorities)". An Van Bay, Nguyen Hai Nam and Cao Lam Anh Page 2 of 2 22 "Dak Nue Commune, Lak District, Dak Lak (Mnong minority)." Bao Huy, Tran Huu Nghi, Nguyen Hai Nam 26 "Phuc Sen Commune, Quang Hoa District, Cao Bang Province (Nung An minority)." Nguyen Huy Dung, Nguyen Hai Nam and Pham Quoc Hung 30 "Doi and Ke Villages, Hien Luong Commune, Da Bac District, Hoa Binh Province (Muong minority)." Vu Long, Nguyen Duy Phu and Cao Lam Anh 33 "Giang Cai Village, Nam Lanh Commune, Van Chan District, Yen Bai Province (Dao minority)." Bui Dinh Toai, Nguyen Phuc Cuong, Vo Thanh Son, Edwin Shanks and Sheelagh O’Reilly 37 "Cu Jiang Commune, Ea Kar District, Dak Lak Province, (Ede minority)." Bao Huy and Tran Huu Nghi 40 "Dak Tover Commune, Chu Pah District, Gia Lai Province (Jarai -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115