View PDF Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
REV Entry List
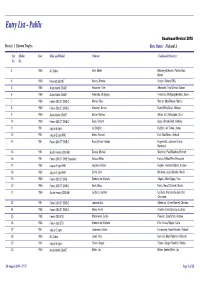
Entry List - Public Goodwood Revival 2018 Race(s): 1 Kinrara Trophy - Race Status: National A Car Shelter Year Make and Model Entrant Confirmed Driver(s) No. No. 3 1963 AC Cobra Hunt, Martin Blakeney-Edwards, Patrick/Hunt, Martin 4 1960 Maserati 3500GT Rosina, Stefano Rosina, Stefano/TBC, 5 1960 Aston Martin DB4GT Alexander, Tom Alexander, Tom/Wilmott, Adrian 6 1960 Aston Martin DB4GT Friedrichs, Wolfgang Friedrichs, Wolfgang/Hadfield, Simon 7 1960 Ferrari 250 GT SWB/C Werner, Max Werner, Max/Werner, Moritz 8 1960 Ferrari 250 GT SWB/C Allemann, Benno Dowd, Mike/Gnani, Michael 9 1960 Aston Martin DB4GT Mosler, Mathias Gillian, G.C./Woodgate, Chris 10 1960 Ferrari 250 GT SWB/C Gaye, Vincent Gaye, Vincent/Reid, Anthony 11 1961 Jaguar E-type Ian Dalglish Dalglish, Ian/Turner, James 12 1961 Jaguar E-type FHC Meins, Richard Huff, Rob/Meins, Richard 14 1961 Ferrari 250 GT SWB/C Racing Team Holland Hugenholtz, John/van Oranje, Bernhard 15 1961 Austin Healey 3000 Mk1 Darcey, Michael Woolmer, Paul/Woolmer, Richard 16 1961 Ferrari 250 GT SWB 'Breadvan' Halusa, Niklas Halusa, Niklas/Pirro, Emanuele 17 1962 Jaguar E-type FHC Hayden, Andrew Hayden, Andrew/Hibberd, Andrew 18 1962 Jaguar E-type FHC Corrie, John Minshaw, Jason/Stretton, Martin 19 1960 Ferrari 250 GT SWB Scuderia del Viadotto Vögele, Alain/Vögele, Yves 20 1960 Ferrari 250 GT SWB/C Devis, Marc Devis, Marc/O'Connell, Martin 21 1960 Austin Healey 3000 Mk1 Le Blanc, Karsten Le Blanc, Karsten/van Lanschot, Christiaen 23 1961 Ferrari 250 GT SWB/C Lanzante Ltd. Ellerbrock, Olivier/Glaesel, Christian -

DOT Number Database Database of Department of Transportation Numbers on Windshields from Various Sources Dot Number Manufacturer Location DOT‐1 SUPERGLASS S.A
DOT Number Database Database of Department of Transportation Numbers on Windshields From Various Sources Dot Number Manufacturer Location DOT‐1 SUPERGLASS S.A. EL TALAR TIGRE BS.AS. ARGENTINA DOT‐2 J‐DAK, INC. SPRINGFIELD TN UNITED STATES DOT‐3 SACOPLAST S.R.L. OTTIGLIO ALESSANDRIA ITALY DOT‐4 SOMAVER AIN SEBAA CASABNLANCA MOROCCO DOT‐5 JIANGUIN JINGEHENG HIGH‐QUAL. DECORATING GLASS WORKS JIANGUIN JIANGSU PROVINCE CHINA DOT‐6 BASKENT GLASS COMPANY SINCAN ANKARA TURKEY DOT‐7 POLPLASTIC SPA DOLO VENEZIA ITALY DOT‐8 CEE BAILEYS #1 MONTEBELLO CA DOT‐9 VIDURGLASS MANBRESA BARCELONA SPAIN DOT‐10 VITRERIE APRIL, INC. P.A.T. MONREAL QUEBEC CANADA DOT‐11 SPECTRA INC. MILWAUKEE WI DOT‐12 DONG SHIN SAFETY GLASS CO., LTD. BOOKILMEON, JEONNAM KOREA DOT‐13 YAU BONG CAR GLASS CO., LTD. ON LOK CHUEN, NEW TERRITORIES HONG KONG DOT‐15 LIBBEY‐OWENS‐FORD CO TOLEDO, OH, USA DOT‐16 HAYES‐ALBION CORPORATION JACKSON, MS, USA DOT‐17 TRIPLEX SAFETY GLASS COMPANY LIMITED BIRMINGHAM, ENGLAND DOT‐18 PPG INDUSTRIES PITTSBURGH, PA, USA DOT‐19 PPG CANADA INC.,DUPLATE DIVISION OSHAWA,ONTARIO, CANADA DOT‐20 ASAHI GLASS CO LTD TOKYO, JAPAN DOT‐21 CHRYSLER CORP DETROIT, MI, USA DOT‐22 GUARDIAN INDUSTRIES CORP AUBURN HILLS, MI, USA DOT‐23 NIPPON SHEET GLASS CO. LTD OSAKA, JAPAN DOT‐24 SPLINTEX BELGE S.A. GILLY, BELGIUM DOT‐25 FLACHGLAS AUTOMOTIVE GmbH WITTEN, GERMANY Page 1 of 27 Dot Number Manufacturer Location DOT‐26 CORNING GLASS WORKS CORNING, NY, USA DOT‐27 SEKURIT SAINT‐GOBAIN DEUTSCHLAND GMBH GERMANY DOT‐32 GLACERIES REUNIES S.A. BELGIUM DOT‐33 LAMINATED GLASS CORPORATION DETROIT, MI, USA DOT‐35 PREMIER AUTOGLASS CORPORATION LANCASTER, OH, USA DOT‐36 SOCIETA ITALIANA VETRO S.P.A. -

1 Information Memorandum Unsecured, Subordinated Perpetual, Listed, Rated Hybrid Securities in the Form of Non Convertible Deben
INFORMATION MEMORANDUM UNSECURED, SUBORDINATED PERPETUAL, LISTED, RATED HYBRID SECURITIES IN THE FORM OF NON CONVERTIBLE DEBENTURES ON A PRIVATE PLACEMENT BASIS Issue Opening Date: 10 May 2011 Issue Closing Date: 11 May 2011 1 Tata Steel Limited A Public Company incorporated under the Indian Companies Act, VI of 1882 Date of Incorporation: 26 August 1907 Registered Office: 3rd floor, Bombay House, 24 Homi Mody Street, Fort, Mumbai 400-001, India Phone No.: 91 22 6665 7279 Fax No.: 91 22 6665 8113 Email: [email protected] Website: www.tatasteel.com INFORMATION MEMORANDUM FOR ISSUE OF UNSECURED, SUBORDINATED, PERPETUAL, LISTED, RATED HYBRID SECURITIES IN THE FORM OF NON CONVERTIBLE DEBENTURES ON A PRIVATE PLACEMENT BASIS INFORMATION MEMORANDUM FOR PRIVATE PLACEMENT OF UNSECURED, SUBORDINATED PERPETUAL, LISTED, RATED HYBRID SECURITIES IN THE FORM OF NON CONVERTIBLE DEBENTURES OF FACE VALUE OF RS.10,00,000/- EACH FOR CASH AT PAR AGGREGATING TO AN AMOUNT OF RS.525 CRORES, WITH GREEN SHOE OPTION (the “SECURITIES”) TO BE ISSUED IN FINANCIAL YEAR 2011-12 GENERAL RISKS General Risks: Investors are advised to read the risk factors carefully before taking an Investment decision in this issue. For taking an investment decision, investors must rely on their own examination of the issuer and the Information Memorandum including the risks involved. The securities have not been recommended or approved by Securities and Exchange Board of India (SEBI) nor does SEBI guarantee the accuracy or adequacy of this Information Memorandum. Special attention -

103Rd Annual Report 2009-2010
A Global Player A Product Pioneer A Corporate Citizen 103rd Annual Report 2009-2010 000_Covers_TSAR'10.indd 2 6/28/10 12:11:26 PM 000_Covers_TSAR'10.indd 3 6/28/10 12:11:40 PM A Global Player >> A Product Pioneer >> A Corporate Citizen It was the best of times, it was the worst of times... Never before has this famous line meant more. The roller coaster ride that the last two years took the global economy through, proved one fact – that a successful enterprise needs to have the resilience to withstand the highs and lows of a future that often comes unheralded. As a global enterprise, Tata Steel was not unaffected by the challenges of the last two years. Yet, the Company demonstrated resilience by taking several proactive initiatives across all geographies. A tough start to the year was balanced by a rebound in the second half, when these initiatives began to pay off. Undeterred by the economic turbulence, the Company continued to place emphasis on working practices in health, safety and corporate citizenship, with specific initiatives taken in all these areas. In addition, a continued focus on engineering solutions for customers, is helping it maintain its position of a product pioneer. Tata Steel believes in staying alert to future opportunities while never letting go of its core values. This is the philosophy that has underpinned its growth over the years and one that remains its key driving force. 001-011_Part01_TSAR10.indd 1 7/7/10 12:55:19 PM Inside the gas holder - part of the BOS gas recovery scheme at Port Talbot, UK. -

Headline Goes Here
PRESSEINFORMATION 25. Juli 2017 Classic Glass for Classic Cars Pilkington Automotive bei den „Classic Days 2017” Pilkington Automotive ist erstmalig zu Gast bei den „Classic Days” vom 4. bis 6. August 2017 auf Schloss Dyck. Das Klassik- und Motorfestival lockt jährlich mehr als 30.000 Besucher nach Jüchen. Pilkington Automotive wird im Rahmen des Festivals seinen besonderen Service für die Liebhaber von Young- und Oldtimern präsentieren: Der Glashersteller produziert auf Kundenwunsch neue Windschutzscheiben für „alte Schätzchen“. Der Clou dabei ist: Die Scheiben aus Verbundsicherheitsglas tragen die klassischen Logos wie XXX oder SIGLA. Sicherheit nach modernem Standard und nostalgisches Flair müssen also kein Widerspruch sein. Die Windschutzscheiben werden in VSG-Qualität (Verbundsicherheitsglas) nach modernen Fertigungstechniken produziert. Verbundsicherheitsglas erhöht die Sicherheit bei der Fahrt mit Young- und Oldtimern. Die Originalscheiben bestehen in der Regel nur aus Einscheibensicherheitsglas. Es lohnt sich deshalb, eine über die Jahre hinweg zerkratzte oder milchig gewordene Originalscheibe durch eine neue Pilkington-Scheibe aus VSG zu ersetzen. Ein Highlight der „Classic Days“ am Stand der Pilkington Automotive Deutschland GmbH (in der Nähe des US- Car-Bereichs beim Miscanthusfeld) wird der Live-Scheibenaustausch bei einem Opel Manta A (Baujahr 1975) sein. Nach Bedarf und Kundenwunsch Pilkington gehört seit 2006 zur NSG Group, einem der weltweit führenden Glashersteller. Das Unternehmen blickt auf eine fast 60-jährige Tradition bei der Produktion von klassischen Windschutzscheiben für den Ersatzteilmarkt zurück. Das Sortiment umfasst beispielsweise Scheiben für Klassiker wie den Aston Martin, das britische Kultauto von 007 James Bond, den Jaguar XKR, für Land Rover, Range Rover oder den Bentley MK IV. Dank einer großen Auswahl an Werkzeugen können Gläser für eine Vielzahl von Young- und Oldtimern produziert werden – bei Bedarf werden auch neue Werkzeuge für die Scheibenproduktion angefertigt. -

Meggitt PLC Annual Report & Accounts 2020
Meggitt PLC Annual Report & Accounts 2020 Introduction Working closely with our Customers, we deliver technologically differentiated systems and products with high certification requirements in aerospace, defence and selected energy markets. Through focusing on engineering and operational excellence, we build broad installed bases of equipment for which we provide through life services and support. Our ambitious and diverse teams act with integrity to create superior and sustainable value for all of our stakeholders. Meggitt PLC Annual Report & Accounts 2020 1 Strategic Report – 1-89 – Report Strategic Strong portfolio Focus on sustainability Strong leadership Diverse end market exposure Next-generation technologies Leading through the with four aligned divisions that create more sustainable pandemic: responding and and efficient aircraft, engines, adapting to the external power and defence systems environment See more on page 20 See more on page 68 See more on page 14 Meggitt PLC 2 Annual Report & Accounts 2020 What’s in the report Contents We deliver innovative solutions Strategic Report for the most demanding 4 Our vision 6 At a glance 8 Chairman’s statement environments. Our differentiated 10 CEO’s statement 14 Our response to COVID-19 products and technologies 16 Market review 20 Strategy 22 Business model satisfy the highest requirements 24 Innovating for the future 28 Strategy in action for product safety, performance 36 Divisional reviews 44 CFO’s review and reliability and we continue 50 Key performance indicators 54 Risk management -

Santos Annual Report 2005
SAN171 WWW Cover 28/3/06 2:14 PM Page 1 SANTOS ANNUAL ANNUAL SANTOS REPORT 2005 Santos Ltd ABN 80 007 550 923 COVER PHOTOGRAPH: CORPORATE DIRECTORY Roger Lewis (right), Mutineer-Exeter Project Close Out Manager, inspecting the MODEC Venture 11 Floating Production Storage and Offtake vessel with a Quality Assurance Engineer. REGISTERED AND HEAD OFFICE Perth Representative office of Santos Ground Floor, Santos House Level 28, Forrest Centre Asia Pacific Pty Ltd in Jakarta 91 King William Street 221 St Georges Terrace Level 9, Ratu Plaza Office Tower Adelaide, South Australia 5000 Perth, Western Australia 6000 Jalan Jendral Sudirman Kav 9 GPO Box 2455 Telephone 08 9460 8900 Jakarta 10270 Indonesia Adelaide, South Australia 5001 Facsimile 08 9460 8971 PO Box 6221, JKS GN Telephone 08 8218 5111 Jakarta 12060 Indonesia Port Bonython Facsimile 08 8218 5274 Telephone 62-21 270 0410 PO Box 344 Facsimile 62-21 720 4503 SHARE REGISTER Whyalla, South Australia 5600 Ground Floor, Santos House Telephone 08 8640 3100 USEFUL EMAIL CONTACTS INSIDE 91 King William Street Facsimile 08 8640 3200 Share register enquiries: Adelaide, South Australia 5000 [email protected] United States of America GPO Box 2455 Santos USA Corp. Investor enquiries: Adelaide, South Australia 5001 10111 Richmond Avenue, Suite 500 [email protected] INTRODUCING SANTOS PRODUCTION STATISTICS BOARD OF DIRECTORS Telephone 08 8218 5111 Houston, Texas 77042 USA Employment enquiries: Company profile and history, and an overview 16 Summary of production results for 2005. 55 Directors’ biographical details. Facsimile 08 8218 5950 Telephone 1-713 986 1700 of Santos’ vision, strategy and values. -
Preliminary Results for the Year Ended 31St March 2021 Robust Performance and Growth Opportunities Driven by Sustainable Solutions
News Release th Thursday 27 May 2021, 7.00 am Preliminary results for the year ended 31st March 2021 Robust performance and growth opportunities driven by sustainable solutions Robert MacLeod, Chief Executive, commented: Our employees have done a tremendous job adapting to new ways of working through COVID-19, whilst still delivering for our customers. It is thanks to their hard work that we have delivered a robust set of results which, in the context of a pandemic, is especially pleasing and testament to their efforts. Following a challenging first half, we recovered strongly in the second half helped by a strong recovery in our end markets and higher precious metal prices. We are delivering our efficiency programme, tightly managing working capital and generating cash from our more established businesses which we are continuing to invest for growth, particularly in battery materials and hydrogen. In the year we made good strategic progress. We began entering into partnerships to advance the commercialisation of eLNO and secured new customer wins in Fuel Cells. Our investment in sustainable technologies builds on our existing expertise and will enable the transformations in transport, energy, decarbonisation of industry and a circular economy that the world needs to reach net zero – transformations that are at the heart of achieving our vision of a cleaner, healthier world for today and future generations. It is in this context that we’ve launched our new sustainability goals which will result in Johnson Matthey being a net zero business by 2040. Our sustainability agenda relates not only to the way we run our own business, but underpins our strategic focus on the current and future technologies we sell to our customers. -

D5.2 Report on ELIPTIC Twin Call's Funded Activities and Outcomes
Report on ELIPTIC Twin call’s funded activities and outcomes Deliverable D5.2 Lisa Bloß / Henning Günter, Rupprecht Authors Consult Status (D: draft; F: final) F Document’s privacy PU (Public: PU; Private: PR) Reviewed by Hendrik Koch, Bremen SUBV; This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 636012. D5.2 Final Twinning Report SUMMARY SHEET Programme Horizon 2020 Contract N. 636012 Project Title Electrification of public transport in cities Acronym ELIPTIC Coordinator Free Hanseatic City Of Bremen Web-site http://www.eliptic-project.eu/ Starting date 1 June 2015 Number of months 36 months Deliverable N. D5.2 Deliverable Title Report on ELIPTIC Twin call’s funded activities and outcomes Milestones - Version 1 Date of issue 11-06-2018 Distribution [Internal/External] External Dissemination level [Public/ Confidential] Public Abstract As part of the take-up process defined by the ELIPTIC project to facilitate wide adoption of the project results by other cities, the ELIPTIC “twinning fund” was set up, which supported the transfer of measures from ELIPTIC partner cities to “twin” cities. The twinning fund was a financing mechanism consisting of a systematic support programme of site visits at ELIPTIC partner use cases, and additionally individual support in the take- up activities of twinning partners. Overall, the ELIPTIC twinning programme was a great success. The programme effectively supported the transfer of measures from ELIPTIC partner cities to twin cities by enabling joint discussions at user fora and workshops, site visits as well as feasibility studies to assess the local possibilities for public transport electrification. -

Pilkington Optiwhite™ and Pilkington Optiwhite™ E
Low-iron clear float glass Pilkington Optiwhite™ and Pilkington Optiwhite™ E Performance data summary Light Solar Radiant Heat Ug-value Product (W/m2K) Transmittance Reflectance Direct Transmittance Reflectance Absorptance Total Transmittance 2 mm 0.92 0.08 0.91 0.08 0.01 0.91 5.9 *3 mm 0.92 0.08 0.91 0.08 0.01 0.91 5.8 *4 mm 0.92 0.08 0.91 0.08 0.01 0.91 5.8 5 mm 0.91 0.08 0.90 0.08 0.02 0.91 5.8 *6 mm 0.91 0.08 0.90 0.08 0.02 0.90 5.7 8 mm 0.91 0.08 0.89 0.08 0.03 0.90 5.7 10 mm 0.91 0.08 0.88 0.08 0.04 0.89 5.6 12 mm 0.91 0.08 0.88 0.08 0.04 0.89 5.5 15 mm 0.90 0.08 0.87 0.08 0.05 0.88 5.5 19 mm 0.90 0.08 0.86 0.08 0.06 0.87 5.3 The above performance data has been determined in accordance with EN 410 and EN 673 based on 90% argon fill. Pilkington Optiwhite™ E is the name for Pilkington Optiwhite™ manufactured in the UK in 4 and 6 mm thicknesses for use in Pilkington energiKare™ energy-efficient glazing and 3 mm for laminates. The performance figures are exactly the same as for Pilkington Optiwhite™ however the colour of the glass as viewed from the edges may not be as clear. -

Chief Executive's Statement
Strategic Report Chief Executive’s statement Using our science to make the world cleaner and healthier As 2018/19 drew to a close, we sat down with Robert MacLeod to ask him about progress during the year, his expectations for 2019/20 and his perspective on JM’s relevance in today’s changing world. How have global megatrends developed for JM over We are therefore expanding our manufacturing capacity the last year? for emission control catalysts with major new plants in Europe, China and India; the plants in Europe and China will come on We have an inspiring vision at JM – for a world that is cleaner line in 2019/20. and healthier, today and for future generations. JM, and what The global automotive industry is going through a once we bring to the world, is arguably more relevant than ever before. in a lifetime transition. Consumer buying patterns are less Over the last 12 months, we have seen public awareness of, predictable and the automotive supply chain, JM included, and action on, the world’s global challenges increase. The next is being more considered in making investments. With less generation is speaking out on global issues such as climate inherent flexibility throughout the supply chain and less change and air pollution, and it is these that JM can, and is, using predictable consumer behaviour, JM is supporting our its science to solve. customers, the car companies, as we navigate this mobility transition together. Focus continues on air quality and emissions – how is JM part of the solution? What impact is that having on your other automotive The need to improve air quality across the world is leading to facing businesses? tighter emissions standards in many countries. -

UK Low Carbon Automotive Directory
UK Low Carbon Automotive Directory UK Trade & Investment is the Government department that helps Contents Introduction 3 UK based companies succeed in an About this directory 4 UK Trade & Investment 5 increasingly global economy. BIS: The Department for Growth 6 Low Carbon Vehicle Partnership 7 Low Carbon Know How 8 Cenex 9 Our range of expert services is Government/NGOs/Trade and Stakeholder Bodies 10 tailored to the needs of individual Academic Research 16 Development 23 businesses to maximise their Production/Manufacture 38 Consultants 58 international success. We provide Training and Qualifications 69 Aftermarket 71 companies with knowledge, advice Matrix of UK Low Carbon Vehicle Capabilities 74 and practical support. UK Low Carbon Automotive Directory Page 2 Introduction Low carbon is the only game in town. This is true From decades of experience, the UK automotive for every nation and for every major industry, not supply chain has built up great business acumen, least the automotive industry which, for decades, contributing to outstanding performance. Partnership in has given us the many and considerable benefits international business is something that comes naturally that come from motorised road transport. And now, to our companies and the UK government supports the automotive sector is again leading the way in that by providing one of the most business-enabled developing low-carbon technologies to ensure that environments in the world. The UK is open for business future generations can continue to enjoy those benefits. and welcomes overseas investment in our low-carbon industries. In 2010, again, the Ernst & Young European The low carbon automotive sector has made Attractiveness Survey found that the UK was the best breathtaking strides in recent years and now promises destination in Europe for foreign direct investment.