PDF Output of CLIC (Clustering by Inferred Co-Expression)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

Role of Mitochondrial Ribosomal Protein S18-2 in Cancerogenesis and in Regulation of Stemness and Differentiation
From THE DEPARTMENT OF MICROBIOLOGY TUMOR AND CELL BIOLOGY (MTC) Karolinska Institutet, Stockholm, Sweden ROLE OF MITOCHONDRIAL RIBOSOMAL PROTEIN S18-2 IN CANCEROGENESIS AND IN REGULATION OF STEMNESS AND DIFFERENTIATION Muhammad Mushtaq Stockholm 2017 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by E-Print AB 2017 © Muhammad Mushtaq, 2017 ISBN 978-91-7676-697-2 Role of Mitochondrial Ribosomal Protein S18-2 in Cancerogenesis and in Regulation of Stemness and Differentiation THESIS FOR DOCTORAL DEGREE (Ph.D.) By Muhammad Mushtaq Principal Supervisor: Faculty Opponent: Associate Professor Elena Kashuba Professor Pramod Kumar Srivastava Karolinska Institutet University of Connecticut Department of Microbiology Tumor and Cell Center for Immunotherapy of Cancer and Biology (MTC) Infectious Diseases Co-supervisor(s): Examination Board: Professor Sonia Lain Professor Ola Söderberg Karolinska Institutet Uppsala University Department of Microbiology Tumor and Cell Department of Immunology, Genetics and Biology (MTC) Pathology (IGP) Professor George Klein Professor Boris Zhivotovsky Karolinska Institutet Karolinska Institutet Department of Microbiology Tumor and Cell Institute of Environmental Medicine (IMM) Biology (MTC) Professor Lars-Gunnar Larsson Karolinska Institutet Department of Microbiology Tumor and Cell Biology (MTC) Dedicated to my parents ABSTRACT Mitochondria carry their own ribosomes (mitoribosomes) for the translation of mRNA encoded by mitochondrial DNA. The architecture of mitoribosomes is mainly composed of mitochondrial ribosomal proteins (MRPs), which are encoded by nuclear genomic DNA. Emerging experimental evidences reveal that several MRPs are multifunctional and they exhibit important extra-mitochondrial functions, such as involvement in apoptosis, protein biosynthesis and signal transduction. Dysregulations of the MRPs are associated with severe pathological conditions, including cancer. -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

Anti-MRPS17 Monoclonal Antibody, Clone FQS23694 (DCABH-6110) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-MRPS17 monoclonal antibody, clone FQS23694 (DCABH-6110) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Product Overview Rabbit monoclonal to MRPS17 Antigen Description Mammalian mitochondrial ribosomal proteins are encoded by nuclear genes and help in protein synthesis within the mitochondrion. Mitochondrial ribosomes (mitoribosomes) consist of a small 28S subunit and a large 39S subunit. They have an estimated 75% protein to rRNA composition compared to prokaryotic ribosomes, where this ratio is reversed. Another difference between mammalian mitoribosomes and prokaryotic ribosomes is that the latter contain a 5S rRNA. Among different species, the proteins comprising the mitoribosome differ greatly in sequence, and sometimes in biochemical properties, which prevents easy recognition by sequence homology. This gene encodes a 28S subunit protein that belongs to the ribosomal protein S17P family. The encoded protein is moderately conserved between human mitochondrial and prokaryotic ribosomal proteins. Pseudogenes corresponding to this gene are found on chromosomes 1p, 3p, 6q, 14p, 18q, and Xq. Immunogen Recombinant fragment within Human MRPS17. The exact sequence is proprietary.Database link: Q9Y2R5 Isotype IgG Source/Host Rabbit Species Reactivity Human Clone FQS23694 Purity Tissue culture supernatant Conjugate Unconjugated Applications IHC-P, WB, ICC/IF, IP Positive Control HeLa, HepG2 and U937 cell lysate; Human kidney and liver tissue; HeLa cells Format Liquid Size 100 μl Buffer pH: 7.2; Preservative: 0.01% Sodium azide; Constituents: 49% PBS, 0.05% BSA, 50% Glycerol 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics All Rights Reserved Preservative 0.01% Sodium Azide Storage Store at +4°C short term (1-2 weeks). -

MPV17L2 Is Required for Ribosome Assembly in Mitochondria Ilaria Dalla Rosa1,†, Romina Durigon1,†, Sarah F
8500–8515 Nucleic Acids Research, 2014, Vol. 42, No. 13 Published online 19 June 2014 doi: 10.1093/nar/gku513 MPV17L2 is required for ribosome assembly in mitochondria Ilaria Dalla Rosa1,†, Romina Durigon1,†, Sarah F. Pearce2,†, Joanna Rorbach2, Elizabeth M.A. Hirst1, Sara Vidoni2, Aurelio Reyes2, Gloria Brea-Calvo2, Michal Minczuk2, Michael W. Woellhaf3, Johannes M. Herrmann3, Martijn A. Huynen4,IanJ.Holt1 and Antonella Spinazzola1,* 1MRC National Institute for Medical Research, Mill Hill, London NW7 1AA, UK, 2MRC Mitochondrial Biology Unit, Wellcome Trust-MRC Building, Hills Road, Cambridge CB2 0XY, UK, 3Cell Biology, University of Kaiserslautern, 67663 Kaiserslautern, Germany and 4Centre for Molecular and Biomolecular Informatics, Radboud University Medical Centre, Geert Grooteplein Zuid 26–28, 6525 GA Nijmegen, Netherlands Received January 15, 2014; Revised May 7, 2014; Accepted May 23, 2014 ABSTRACT INTRODUCTION MPV17 is a mitochondrial protein of unknown func- The mammalian mitochondrial proteome comprises 1500 tion, and mutations in MPV17 are associated with or more gene products. The deoxyribonucleic acid (DNA) mitochondrial deoxyribonucleic acid (DNA) mainte- inside mitochondria DNA (mtDNA) contributes only 13 ∼ nance disorders. Here we investigated its most sim- of these proteins, and they make up 20% of the subunits ilar relative, MPV17L2, which is also annotated as of the oxidative phosphorylation (OXPHOS) system, which produces much of the cells energy. All the other proteins a mitochondrial protein. Mitochondrial fractionation -

Transcriptome Analysis of Induced Pluripotent Stem Cells from Monozygotic Twins Discordant for Trisomy 21
Genomics Data 2 (2014) 226–229 Contents lists available at ScienceDirect Genomics Data journal homepage: http://www.journals.elsevier.com/genomics-data/ Data in Brief Data in brief: Transcriptome analysis of induced pluripotent stem cells from monozygotic twins discordant for trisomy 21 Youssef Hibaoui a,b,IwonaGrada, Audrey Letourneau b, Federico A. Santoni b, Stylianos E. Antonarakis b,c,⁎, Anis Feki a,d,⁎⁎ a Stem Cell Research Laboratory, Department of Obstetrics and Gynecology, Geneva University Hospitals, 30 bd de la Cluse, CH-1211 Geneva, Switzerland b Department of Genetic Medicine and Development, University of Geneva Medical School and Geneva University Hospitals, 1 rue Michel-Servet, CH-1211 Geneva, Switzerland c iGE3 Institute of Genetics and Genomics of Geneva, University of Geneva, Switzerland d Department of Obstetrics and Gynecology, HFR Fribourg—Hôpital cantonal, Chemin des Pensionnats 2-6, Case postale 1708 Fribourg, Switzerland article info abstract Article history: Down syndrome (DS, trisomy 21), is the most common viable chromosomal disorder, with an incidence of 1 in Received 7 July 2014 800 live births. Its phenotypic characteristics include intellectual impairment and several other developmental Received in revised form 22 July 2014 abnormalities, for the majority of which the pathogenetic mechanisms remain unknown. In this “Data in Brief” Accepted 27 July 2014 paper, we sum up the whole genome analysis by mRNA sequencing of normal and DS induced pluripotent Available online 1 August 2014 stem cells that was recently published by Hibaoui et al. in EMBO molecular medicine. © 2014 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license Keywords: Induced pluripotent stem cells (http://creativecommons.org/licenses/by-nc-nd/3.0/). -

Cardiac SARS‐Cov‐2 Infection Is Associated with Distinct Tran‐ Scriptomic Changes Within the Heart
Cardiac SARS‐CoV‐2 infection is associated with distinct tran‐ scriptomic changes within the heart Diana Lindner, PhD*1,2, Hanna Bräuninger, MS*1,2, Bastian Stoffers, MS1,2, Antonia Fitzek, MD3, Kira Meißner3, Ganna Aleshcheva, PhD4, Michaela Schweizer, PhD5, Jessica Weimann, MS1, Björn Rotter, PhD9, Svenja Warnke, BSc1, Carolin Edler, MD3, Fabian Braun, MD8, Kevin Roedl, MD10, Katharina Scher‐ schel, PhD1,12,13, Felicitas Escher, MD4,6,7, Stefan Kluge, MD10, Tobias B. Huber, MD8, Benjamin Ondruschka, MD3, Heinz‐Peter‐Schultheiss, MD4, Paulus Kirchhof, MD1,2,11, Stefan Blankenberg, MD1,2, Klaus Püschel, MD3, Dirk Westermann, MD1,2 1 Department of Cardiology, University Heart and Vascular Center Hamburg, Germany. 2 DZHK (German Center for Cardiovascular Research), partner site Hamburg/Kiel/Lübeck. 3 Institute of Legal Medicine, University Medical Center Hamburg‐Eppendorf, Germany. 4 Institute for Cardiac Diagnostics and Therapy, Berlin, Germany. 5 Department of Electron Microscopy, Center for Molecular Neurobiology, University Medical Center Hamburg‐Eppendorf, Germany. 6 Department of Cardiology, Charité‐Universitaetsmedizin, Berlin, Germany. 7 DZHK (German Centre for Cardiovascular Research), partner site Berlin, Germany. 8 III. Department of Medicine, University Medical Center Hamburg‐Eppendorf, Germany. 9 GenXPro GmbH, Frankfurter Innovationszentrum, Biotechnologie (FIZ), Frankfurt am Main, Germany. 10 Department of Intensive Care Medicine, University Medical Center Hamburg‐Eppendorf, Germany. 11 Institute of Cardiovascular Sciences, -

Using Edsurvey to Analyze NCES Data: an Illustration of Analyzing NAEP Primer
Using EdSurvey to Analyze NCES Data: An Illustration of Analyzing NAEP Primer Developed by Michael Lee, Paul Bailey, Ahmad Emad, Ting Zhang, Trang Nguyen, and Jiao Yu*† February 21, 2020 Overview of the EdSurvey Package National Assessment of Educational Progress (NAEP) datasets from the National Center for Education Statistics (NCES) require special statistical methods to analyze. Because of their scope and complexity, the EdSurvey package gives users functions to perform analyses that account for both complex sample survey designs and the use of plausible values. The EdSurvey package also seamlessly takes advantage of the LaF package to read in data only when required for an analysis. Users with computers that have insuÿcient memory to read in entire NAEP datasets can still do analyses without having to write special code to read in just the appropriate variables. This situation is addressed directly in the EdSurvey package—behind the scenes and without any special tuning by the user. Vignette Outline This vignette will describe the basics of using the EdSurvey package for analyzing NAEP data as follows. • Notes – Additional resources – Vignette notation – Software requirements • Setting up the environment for analyzing NCES data – Installing and loading EdSurvey – Philosophy of Conducting Analyses Using the EdSurvey Package – Downloading data – Reading in data – Getting to know the data format – Removing special values • Explore Variable Distributions with summary2 • Subsetting the data • Retrieving data for further manipulation with getData *This publication was prepared for NCES under Contract No. ED-IES-12-D-0002 with the American Institutes for Research. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. -
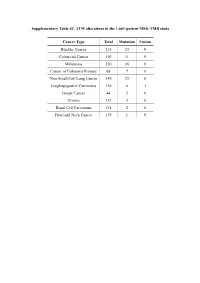
Supplementary Table S1. ATM Alterations in the 1,661-Patient MSK-TMB Study
Supplementary Table S1. ATM alterations in the 1,661-patient MSK-TMB study Cancer Type Total Mutation Fusion Bladder Cancer 215 23 0 Colorectal Cancer 110 11 0 Melanoma 320 26 0 Cancer of Unknown Primary 88 7 0 Non-Small Cell Lung Cancer 350 23 0 Esophagogastric Carcinoma 126 6 1 Breast Cancer 44 2 0 Glioma 117 3 0 Renal Cell Carcinoma 151 2 0 Head and Neck Cancer 139 1 0 Supplementary Table S2. ATM alternations in the 10,945-patient MSK-IMPACT study Cancer Type Total Mutation Deep deletion Amplification Multi-alterations Fusion Small Bowl Cancer 35 7 0 0 0 0 Skin Cancer, Non-Melaoma 148 19 1 0 0 0 Bladder Cancer 423 45 2 0 0 0 Endometrial Cancer 218 24 0 0 0 0 Hepatobiliary Cancer 355 27 0 0 0 0 Colorectal Cancer 1007 75 0 0 0 1 Mature B-Cell Neoplasms 134 8 0 0 2 0 Non-Small Cell Lung Cancer 1668 120 0 2 1 0 Melanoma 365 23 0 0 0 0 Appendiceal Cancer 79 4 0 0 0 0 Small Cell Lung Cancer 82 4 0 0 0 0 Prostate Cancer 717 25 7 1 0 0 Histiocytosis 22 1 0 0 0 0 Salivary Gland Cancer 114 5 0 0 0 0 Thyroid Cancer 231 10 0 0 0 0 Cancer of Unknown Primary 186 8 0 0 0 0 Breast Cancer 1324 48 3 2 0 0 Adrenocortical Carcinoma 25 1 0 0 0 0 Mature T and NK Neoplasms 29 1 0 0 0 0 Renal Cell Carcinoma 361 12 0 0 0 0 Head and Neck Cancer 186 14 0 0 0 1 Pancreatic Cancer 502 69 7 2 0 0 Glioma 553 14 1 0 0 0 Soft Tissue Sarcoma 443 13 1 0 0 0 Esophagogastric Cancer 341 8 1 0 0 0 Peripheral Nervous System 80 0 2 0 0 0 Germ Cell Tumor 288 2 0 1 0 1 Ovarian Can 244 2 1 0 0 0 Uterine Sarcoma 93 1 0 0 0 0 Mesothelioma 107 1 0 0 0 0 Bone Cancer 134 1 0 0 0 0 Gastrointestinal Stromal Ca 137 0 0 0 0 1 Supplementary Table S3. -

Inhibition of the MID1 Protein Complex
Matthes et al. Cell Death Discovery (2018) 4:4 DOI 10.1038/s41420-017-0003-8 Cell Death Discovery ARTICLE Open Access Inhibition of the MID1 protein complex: a novel approach targeting APP protein synthesis Frank Matthes1,MoritzM.Hettich1, Judith Schilling1, Diana Flores-Dominguez1, Nelli Blank1, Thomas Wiglenda2, Alexander Buntru2,HannaWolf1, Stephanie Weber1,InaVorberg 1, Alina Dagane2, Gunnar Dittmar2,3,ErichWanker2, Dan Ehninger1 and Sybille Krauss1 Abstract Alzheimer’s disease (AD) is characterized by two neuropathological hallmarks: senile plaques, which are composed of amyloid-β (Aβ) peptides, and neurofibrillary tangles, which are composed of hyperphosphorylated tau protein. Aβ peptides are derived from sequential proteolytic cleavage of the amyloid precursor protein (APP). In this study, we identified a so far unknown mode of regulation of APP protein synthesis involving the MID1 protein complex: MID1 binds to and regulates the translation of APP mRNA. The underlying mode of action of MID1 involves the mTOR pathway. Thus, inhibition of the MID1 complex reduces the APP protein level in cultures of primary neurons. Based on this, we used one compound that we discovered previously to interfere with the MID1 complex, metformin, for in vivo experiments. Indeed, long-term treatment with metformin decreased APP protein expression levels and consequently Aβ in an AD mouse model. Importantly, we have initiated the metformin treatment late in life, at a time-point where mice were in an already progressed state of the disease, and could observe an improved behavioral phenotype. These 1234567890 1234567890 findings together with our previous observation, showing that inhibition of the MID1 complex by metformin also decreases tau phosphorylation, make the MID1 complex a particularly interesting drug target for treating AD. -

Supplementary Dataset S2
mitochondrial translational termination MRPL28 MRPS26 6 MRPS21 PTCD3 MTRF1L 4 MRPL50 MRPS18A MRPS17 2 MRPL20 MRPL52 0 MRPL17 MRPS33 MRPS15 −2 MRPL45 MRPL30 MRPS27 AURKAIP1 MRPL18 MRPL3 MRPS6 MRPS18B MRPL41 MRPS2 MRPL34 GADD45GIP1 ERAL1 MRPL37 MRPS10 MRPL42 MRPL19 MRPS35 MRPL9 MRPL24 MRPS5 MRPL44 MRPS23 MRPS25 ITB ITB ITB ITB ICa ICr ITL original ICr ICa ITL ICa ITL original ICr ITL ICr ICa mitochondrial translational elongation MRPL28 MRPS26 6 MRPS21 PTCD3 MRPS18A 4 MRPS17 MRPL20 2 MRPS15 MRPL45 MRPL52 0 MRPS33 MRPL30 −2 MRPS27 AURKAIP1 MRPS10 MRPL42 MRPL19 MRPL18 MRPL3 MRPS6 MRPL24 MRPS35 MRPL9 MRPS18B MRPL41 MRPS2 MRPL34 MRPS5 MRPL44 MRPS23 MRPS25 MRPL50 MRPL17 GADD45GIP1 ERAL1 MRPL37 ITB ITB ITB ITB ICa ICr original ICr ITL ICa ITL ICa ITL original ICr ITL ICr ICa translational termination MRPL28 MRPS26 6 MRPS21 PTCD3 C12orf65 4 MTRF1L MRPL50 MRPS18A 2 MRPS17 MRPL20 0 MRPL52 MRPL17 MRPS33 −2 MRPS15 MRPL45 MRPL30 MRPS27 AURKAIP1 MRPL18 MRPL3 MRPS6 MRPS18B MRPL41 MRPS2 MRPL34 GADD45GIP1 ERAL1 MRPL37 MRPS10 MRPL42 MRPL19 MRPS35 MRPL9 MRPL24 MRPS5 MRPL44 MRPS23 MRPS25 ITB ITB ITB ITB ICa ICr original ICr ITL ICa ITL ICa ITL original ICr ITL ICr ICa translational elongation DIO2 MRPS18B MRPL41 6 MRPS2 MRPL34 GADD45GIP1 4 ERAL1 MRPL37 2 MRPS10 MRPL42 MRPL19 0 MRPL30 MRPS27 AURKAIP1 −2 MRPL18 MRPL3 MRPS6 MRPS35 MRPL9 EEF2K MRPL50 MRPS5 MRPL44 MRPS23 MRPS25 MRPL24 MRPS33 MRPL52 EIF5A2 MRPL17 SECISBP2 MRPS15 MRPL45 MRPS18A MRPS17 MRPL20 MRPL28 MRPS26 MRPS21 PTCD3 ITB ITB ITB ITB ICa ICr ICr ITL original ITL ICa ICa ITL ICr ICr ICa original -

The Pennsylvania State University the Graduate School Eberly
The Pennsylvania State University The Graduate School Eberly College of Science REGULATION OF MITOCHONDRIAL TRANSLATION AND OXIDATIVE PHOSPHORYLATION THROUGH REVERSIBLE ACETYLATION A Dissertation in Biochemistry, Microbiology and Molecular Biology by Hüseyin Çimen 2012 Hüseyin Çimen Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2012 The Dissertation of Hüseyin Çimen was reviewed and approved* by the following: Emine C. Koc Assistant Professor of Biochemistry and Molecular Biology Dissertation Co-adviser Co-chair of Committee Hasan Koc Assistant Professor of Natural Sciences Dissertation Co-adviser Co-chair of Committee Craig E. Cameron Paul Berg Professor of Biochemistry and Molecular Biology Associate Department Head for Research and Graduate Education Joseph C. Reese Professor of Biochemistry and Molecular Biology Teh-hui Kao Professor of Biochemistry and Molecular Biology Tae-Hee Lee Assistant Professor of Chemistry and the Huck Institute of the Life Sciences Craig E. Cameron Paul Berg Professor of Biochemistry and Molecular Biology Associate Department Head of the Department of Biochemistry and Molecular Biology iii ABSTRACT In a eukaryotic cell, mitochondria provide energy in the form of ATP through oxidative phosphorylation (OXPHOS), which consists of five electron transport chain complexes embedded in the inner membrane of mitochondria. Human mitochondria have their own genome and transcription/translation system to synthesize mitochondrially encoded thirteen proteins of respiratory chain complexes. We investigated how acetylation of ribosomal proteins regulates translation and energy production in mitochondria since reversible acetylation of mitochondrial proteins was found to be critical for maintaining energy homeostasis. We identified mitochondrial ribosomal protein L10 (MRPL10) as the major acetylated ribosomal protein in mammalian mitochondria with two-dimensional gel electrophoresis followed by tandem mass spectrometry and immunoblotting analyses.