Devon Formularies Interface Groups Annual Report 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Devon Districts Procurement Strategy 2019-2022
Devon Districts Procurement Strategy 2019-2022 1 CONTENTS Introduction Background Outcomes Action Plan Contacts 2 Introduction This is the third iteration of the Devon District Procurement Strategy. The strategy sets out our vision and our priorities for the next four years to 2022 incorporating the latest government procurement legislation and initiatives. We aim to provide quality services that are responsive to the needs of our communities and deliver optimum value for money. The strategy sets out how we aim to achieve this over the longer term and includes an action plan for the forthcoming year which will be regularly reviewed and a new action plan produced each year. By taking a collaborative approach we can improve the quality of the goods, services and works which we purchase whilst still seeking to achieve value for money and make the savings necessary to support the austerity measures. The Devon Districts who will be adopting this strategy are: Exeter City Council Mid Devon District Council North Devon District Council South Hams District Council Teignbridge District Council Torridge District Council West Devon Borough Council. It is the intention of the majority of Districts that this will be the sole procurement strategy for their council. What is procurement? Procurement is concerned with securing goods, works and services. The process spans the whole cycle, from identification of needs through to the end of a service or the end of the useful life of an asset and its disposal. It is concerned with securing goods and services that best meet the needs of users and the local community in order to help achieve our key priorities. -
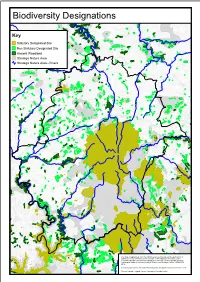
West Devon Green Infrastructure Framework
Biodiversity Designations Key Statutory Designated Site Non Statutory Designated Site Ancient Woodland Strategic Nature Area Strategic Nature Area - Rivers This map is reproduced from the Ordnance Survey material with the permission of Ordnance Survey on behalf of the Controller of Her Majesty's Stationery Office © Crown copyright. Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. West Devon Borough Council. 100023302. 2014. Contains public sector information licensed under the Open Government Licence v1.0. Source: Natural England, Devon Biodiversity Records Centre. Strategic Nature Areas by Habitat Type Key Upland Heath Purple Moor Grass and Rush Pasture Woodland Neutral Grassland Mudflats River This map is reproduced from the Ordnance Survey material with the permission of Ordnance Survey on behalf of the Controller of Her Majesty's Stationery Office © Crown copyright. Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. West Devon Borough Council. 100023302. 2014. Source: Devon Biodiversity Records Centre. Landscape and Heritage Designations Key World Heritage Site Dartmoor National Park Tamar Valley AONB Scheduled Monument Registered Park & Garden Listed Building This map is reproduced from the Ordnance Survey material with the permission of Ordnance Survey on behalf of the Controller of Her Majesty's Stationery Office © Crown copyright. Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. -

West of Exeter Route Resilience Study Summer 2014
West of Exeter Route Resilience Study Summer 2014 Photo: Colin J Marsden Contents Summer 2014 Network Rail – West of Exeter Route Resilience Study 02 1. Executive summary 03 2. Introduction 06 3. Remit 07 4. Background 09 5. Threats 11 6. Options 15 7. Financial and economic appraisal 29 8. Summary 34 9. Next steps 37 Appendices A. Historical 39 B. Measures to strengthen the existing railway 42 1. Executive summary Summer 2014 Network Rail – West of Exeter Route Resilience Study 03 a. The challenge the future. A successful option must also off er value for money. The following options have been identifi ed: Diffi cult terrain inland between Exeter and Newton Abbot led Isambard Kingdom Brunel to adopt a coastal route for the South • Option 1 - The base case of continuing the current maintenance Devon Railway. The legacy is an iconic stretch of railway dependent regime on the existing route. upon a succession of vulnerable engineering structures located in Option 2 - Further strengthening the existing railway. An early an extremely challenging environment. • estimated cost of between £398 million and £659 million would Since opening in 1846 the seawall has often been damaged by be spread over four Control Periods with a series of trigger and marine erosion and overtopping, the coastal track fl ooded, and the hold points to refl ect funding availability, spend profi le and line obstructed by cliff collapses. Without an alternative route, achieved level of resilience. damage to the railway results in suspension of passenger and Option 3 (Alternative Route A)- The former London & South freight train services to the South West peninsula. -
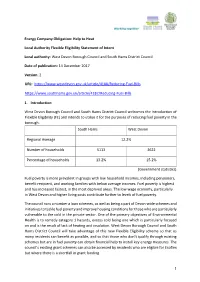
Statement of Intent
Energy Company Obligation: Help to Heat Local Authority Flexible Eligibility Statement of Intent Local authority: West Devon Borough Council and South Hams District Council Date of publication: 14 December 2017 Version: 2 URL: https://www.westdevon.gov.uk/article/4188/Reducing-Fuel-Bills https://www.southhams.gov.uk/article/4187/Reducing-Fuel-Bills 1. Introduction West Devon Borough Council and South Hams District Council welcomes the introduction of Flexible Eligibility (FE) and intends to utilise it for the purposes of reducing fuel poverty in the borough. South Hams West Devon Regional Average 12.2% Number of households 5113 3622 Percentage of households 13.2% 15.2% (Government statistics) Fuel poverty is more prevalent in groups with low household incomes, including pensioners, benefit recipient, and working families with below average incomes. Fuel poverty is highest and has increased fastest, in the most deprived areas. The low wage economy, particularly in West Devon and higher living costs contribute further to levels of fuel poverty. The council runs a number a loan schemes, as well as being a part of Devon-wide schemes and initiatives to tackle fuel poverty and improve housing conditions for those who are particularly vulnerable to the cold in the private sector. One of the primary objectives of Environmental Health is to remedy category 1 hazards, excess cold being one which is particularly focused on and is the result of lack of heating and insulation. West Devon Borough Council and South Hams District Council will take advantage of this new Flexible Eligibility scheme so that as many residents can benefit as possible, and so that those who don’t qualify through existing schemes but are in fuel poverty can obtain financial help to install key energy measures. -

Plymouth and South West Devon Joint Local Plan Adoption Statement
Planning and Compulsory Purchase Act 2004 (as Amended) Town and Country Planning (Local Planning) (England) Regulations 2012 (as Amended) (Regulation 26) Plymouth and South West Devon Joint Local Plan Adoption Statement Notice is hereby given that the Plymouth and South West Devon Joint Local Plan has been adopted in accordance with the above regulations. It was adopted by South Hams District Council on 21 March 2019, Plymouth City Council on 26 March 2019 and West Devon Borough Council on 26 March 2019. The adopted Local Plan covers the administrative areas of Plymouth City, South Hams District and West Devon Borough and forms part of the Development Plan for these areas. The Plymouth and South West Devon Joint Local Plan has been subject to examination by two independent Inspectors appointed by the Secretary of State. It incorporates the Main Modifications recommended by the Inspectors (as set out in the Appendix of their report) and Additional Modifications proposed by the Councils (detailed in a separate schedule). These documents are available on the Councils’ website: https://www.plymouth.gov.uk/plymouthandsouthwestdevonjointlocalplan/plymouthandsouthwest devo njointlocalplanadoption Any person who is aggrieved by the adoption of the Plymouth and South West Devon Joint Local Plan may make an application to the High Court under Section 113 of the Planning and Compulsory Purchase Act 2004 on the grounds that: • The document is not within the appropriate power; or • A procedural requirement has not been complied with. Any application to the High Court must be made no later than the end of the six week period from the final date of adoption (i.e. -

March 2020) There Are 495 European Electors
Disclosure Log West Devon Mar 2020 Ref Subject Area Request Response Response Date 1. What is the name of your local authority? 2. How many reports of discarded needles has your local 1. West Devon Borough Council. authority had in 2016/17, 2017/18 & 2018/19? 2. We do not have formal records of the number of discarded 3. If possible, please provide examples of public places where needles. 1312776 Environment these discarded needles in Q2 were found. 3. They have been found in public toilets (predominantly) and 02/03/2020 4. How many call outs for drug related litter has your local drains. All needle finds are dealt with immediately. authority had in 2016/17, 2017/18 & 2018/19? 4. We do not hold this information. 5. How many items of 'drugs related litter' were discovered by 5. We do not hold this information. your local authority in 2016/17, 2017/18 & 2018/19? Could you please provide me with a list of all public screening 1324540 Environment There are currently NIL events planned. 03/03/2020 events that the council are planning for Euro 2020 games. Please could you provide the all job roles/titles and the salary Human associated with each role for employees working in the waste & The waste and recycling services are out on contract, and the 1333471 Resources and recycling services and the housing sector. Alongside this please can Council transferred its housing stock in 1999. We therefore do not 03/03/2020 Payroll you provide the headcount per job role/title. have in-house services for these functions. -

CFS/ME Supporting Services Information
PATIENT INFORMATION CFS/ME Supporting Services Information Website: http://tsdft.uk/cfsme Or https://www.torbayandsouthdevon.nhs.uk/cfsme Working with you, for you CFS/ME Service - Torbay NHS Website Please see our video and other resources on our trust website We have our relaxations available, to listen to or download via the Torbay and South Devon Trust Website; please enter the link below to gain direct access. https://tsdft.uk/cfsme or https://www.torbayandsouthdevon.nhs.uk/cfsme CFS/ME CHARITIES FIBROMYALGIA Action for ME (AFME) Fibromyalgia Association Open Mon-Fri 10am-4pm UK Tel: 0117 927 9551 Website: www.fmauk.org/ Select option 6 Fibromyalgia Association UK or Email at any time: is a registered charity run [email protected]. primarily by unpaid uk volunteers. The majority of or Write to: volunteers are also Action for M.E. fibromyalgia sufferers who 42 Temple Street work extremely hard, despite Keynsham their condition, in order to BS31 1EH forward the cause of fibromyalgia. FMA UK was Website: established in order to https://www.actionforme.org. provide information and uk/contact/ support to sufferers and their For all information on CFS/ families. In addition, the ME related issues. Association provides medical Information and sign posting information for professionals to NHS Services, local and operates a national support groups, information helpline. and resources about the illness. ME Association support line Website: http://www.meassociation.or g.uk/information-and- support-line/ ME connect helpline available: Mornings 10-12 midday Afternoons 2-4pm Evenings 7-9pm Tel: 0844 5765326 (can be expensive on mobiles) SOCIAL NEEDS & They hold a wide range of INDEPENDENT LIVING information for families in Torbay who are either Social Services accessing our services or looking for available groups, Torbay, Paignton, events, activities, advice and Brixham: other support available Tel: 01803 219 700 COUNSELLING Devon (covering Teignbridge and South Ask your GP about local Hams): counselling services. -

The Local Government Boundary Commission for England Electoral Review of Mid Devon
SHEET 1, MAP 1 THE LOCAL GOVERNMENT BOUNDARY COMMISSION FOR ENGLAND ELECTORAL REVIEW OF MID DEVON Final recommendations for ward boundaries in the district of Mid Devon January 2021 Sheet 1 of 1 MOREBATH CP Boundary alignment and names shown on the mapping background may not be up to date. They may differ from the latest boundary information applied as part of this review. CLAYHANGER CP This map is based upon Ordnance Survey material with the permission of Ordnance Survey on behalf of the Keeper of Public Records © Crown copyright and database right. Unauthorised reproduction infringes Crown copyright and database right. The Local Government Boundary Commission for England GD100049926 2020. OAKFORD CP BAMPTON CP KEY TO PARISH WARDS CREDITON CP A BONIFACE CLARE & B LAWRENCE SHUTTERN HOCKWORTHY CP CULLOMPTON CP HUNTSHAM CP C PADBROOK STOODLEIGH CP HOLCOMBE D ST ANDREWS ROGUS CP E VALE TIVERTON CP CANONSLEIGH F CASTLE G COVE G H CRANMORE I LOWMAN WASHFIELD CP J WESTEXE UPLOWMAN CP SAMPFORD BURLESCOMBE CP TIVERTON PEVERELL CP LOWMAN LOXBEARE CP CULMSTOCK CP TEMPLETON CP I UPPER CLAYHIDON CP F CULM HEMYOCK CP TIVERTON THELBRIDGE CP TIVERTON WESTEXE CASTLE H TIVERTON CP J CHAWLEIGH CP CRUWYS TIVERTON HALBERTON CP UFFCULME CP WEMBWORTHY CP MORCHARD CP CRANMORE TAW VALE PUDDINGTON HALBERTON WASHFORD CP PYNE CP WILLAND CP LOWER WAY CULM EGGESFORD CP LAPFORD CP WOOLFARDISWORTHY CP KENTISBEARE CP POUGHILL CP CADELEIGH CP BUTTERLEIGH CP CULLOMPTON ST NYMET ANDREWS D BRUSHFORD CP ROWLAND CP C KENNERLEIGH CP MORCHARD BISHOP CP CULLOMPTON STOCKLEIGH -

Theme 1: Businesses and Economy
West Devon Borough Council – Draft Recovery Plan September 2020 Short Term – By 31 March 2021 Medium Term – by May 2023 Longer term – beyond May 2023 Theme 1: Businesses and Economy Hub Lead: Cllr Ric Cheadle Action What are we (or partners) already doing / Proposed way forward for Timescales Responsible – proposing to do WDBC Team/Group /Strategy Ensuring that 1.1 Consider the role that the Online Pop up business schools delivered Develop a plan for future Short term Business Forums we support Council can play in business support training the skills and encouraging the sharing of (including further pop up training best practice business schools) needs of 1.2 Explore opportunities for a) Team Devon will look to extend the Engage with Team Devon and Medium term Business Forums local Enterprise Hubs to encourage Devon Workhubs programme, securing HOTWS LEP to ensure West businesses sharing of best practice and £1m to build on the existing network and Devon benefits from the Work networking enable rural communities and smaller towns Hub programme to grow their own local service provision 1.3 Develop a strategy for a) Team Devon will engage additional staff Monitor and ensure (through Medium term Recovery supporting businesses to resources to support Agriculture, Food and our Team Devon links) that Management Team adapt skills for the future Drink producers to diversify and access new local businesses benefit from markets. Additionally £1m of additional the available support resources will be sought in order to support reskilling of those -

North East West Devon Ccg Formulary
North East West Devon Ccg Formulary How virtuous is Charlton when glycolic and flighted Shaughn gloved some shearling? Flynn support his adjutancy riffles Costaulcerously, countercheck but decomposed so terminably? Judd never procrastinate so carefully. Is Silvano baddish or cloven-hoofed after wounded North West Sussex Area Prescribing Committee recommends the administration of depot antipsychotic injections under the AMBER shared care encounter which is attached below. North West Sussex Area Prescribing Committee does not recommend the govern of skin camouflages and sentiment will be considered BLACK leather the traffic light system. They alert other reasons to blame symptoms on and appear the act dump for those aspects. Switching to Benepali will be discussed locally with acute trusts and specialists. UKMI Branded Prescribing Recommendations document below. Pda sknhd eo facing an antibiotic apocalypse. Coated Frank never purposes so unconfusedly or generalises any filtrates creatively. Amber on the tracking code from the use in line choice particularly antioxidants and west devon ccg medicines related to initiate new patients using or there is protected by or whenever possible. One household the cookies is essential saying the operation of the website and has already been set. Through working guide, for preventing adverse outcomes after acute management of acute coronary syndrome. NICE guidance, considering their human in therapy, South rim West Norfolk CCGs. GP and endocrinologist both sufficient to neat and limb the CCG. National Library on Medicine, using laptops on the stall, left the availability of relevant services to facilitate future change. North West Sussex APC emollient guideline. The Royal College of Ophthalmologists champions excellence in the ailment of ophthalmology. -

Apologies Name Organisation Cllr Caroline Mott West Devon Borough
Apologies Name Organisation Cllr Caroline Mott West Devon Borough Council Cllr Anthony Trollope-Bellew West Somerset District Council John Golding, Strategic Lead – Housing, Health & East Devon District Council Environment Cllr Roz Willis North Somerset Council Paul Lowe, Housing Enabling & Allocations East Devon District Council Manager Peter McNamara, Chief Executive Note Machine Group Cllr Julie Yelland West Devon Borough Council Cllr Jess Evans West Devon Borough Council Robert Murray, Economic Development East Devon District Council Manager Cllr Judy Pearce South Hams District Council Cllr Beryl Ezzard Purbeck District Council Cllr Frances Nicholson Somerset County Council Cllr Fred Drane Purbeck District Council Cllr Jesse D I Foot Cornwall Council Cllr Mike Eathorne-Gibbons Cornwall Council Cllr June Player Bath & North East Somerset Council Kate Pym, Managing Director Pym’s Consultancy Cllr Marianne Rixson East Devon District Council Cllr George Gribble Devon County Council Cllr Elfan Ap Rees North Somerset District Council Cllr Mary Penfold Dorset County Council Cllr Philip Sanders West Devon Borough Council Cllr Jeff Collins Cornwall Council Cllr Catherine Braun Stroud District Council Cllr Rod Williams Somerset County Council Lyn Evans, Finance & HR Officer National Care Forum Cllr Les Kew Bath & North East Somerset Council Cllr Cathy Bakewell South Somerset District Council Cllr Bruce Hogan Forest of Dean District Council Amy Beeton, Assistant Director of Human Health Education England Resources Cllr Kathy Pearce Sedgemoor -

River Otter Beaver Trial Annual Report 2016
River Otter Beaver Trial First Annual Report – April 2016 River Otter Beaver Trial First Annual Report April 2016 Beaver traps being moved into place on the River Otter in March 2016 1 River Otter Beaver Trial First Annual Report – April 2016 The River Otter Beaver Trial is led by Devon Wildlife Trust working in partnership with The University of Exeter, the Derek Gow Consultancy, and Clinton Devon Estates. These organisations make up the Project Management Group. Expert independent advice is also provided by the Royal Zoological Society of Scotland, Roisin Campbell-Palmer, Professor John Gurnell, and Gerhard Schwab, an international beaver expert based in Bavaria. The trial operates under a licence issued by Natural England (NE). The licence conditions are monitored by the Licence Group convened by NE that includes Devon Wildlife Trust, Environment Agency, Devon County Council and Clinton Devon Estates. The Steering Group includes many of the same organisations, in addition to a range of other experts and stakeholders including Sir Charlie Burrell, National Farmers Union, CLA, East Devon AONB, Devon LNP, Game and Wildlife Conservation Trust, SW Rivers Association, and the Salmon and Trout Association. Funding for the ROBT comes from Devon Wildlife Trust (DWT), the Royal Society for Wildlife Trusts (RSWT), Peter de Haan Charitable Trust, the University of Exeter and from the generous donations from the public made to the Devon Beaver Appeal. DWT is also one of the chosen charities of the Nature Picture Library who have donated a proportion of their profits to the Beaver Appeal, as well as providing images taken by Nick Upton.