Towards Risk-Based Surveillance of African Swine Fever in Switzerland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

J I W November, 1935
LANGLER -...-: ••. _•••• ••• Zgvp": 'k'^ m J i w \M I 'Ml November, 1935 OFFICIAL STATE NOVEMBER, 1935 PUBLICATION ^ANGLER? Vol. 4 No. 11 ,<>. COMMONWEALTH OF PENNSYLVANIA PUBLISHED MONTHLY BOARD OF FISH COMMISSIONERS by the PENNSYLVANIA BOARD OF FISH COMMISSIONERS l8il £3 E3 ES HP OLIVER M. DEIBLER Five cents a copy — 50 cents a year Commissioner of Fisheries C. R. BULLER Deputy Commissioner of Fisheries szxx Pleasant Mount E3S2E3 ALEX P. SWEIGART, Editor South Office Bldg.. Harrisburg, Pa. MEMBERS OF BOARD OLIVER M. DEIBLER, Chairman Greensburg E3J3S DAN R. SCHNABEL Johnstown LESLIE W. SEYLAR NOTE McConnellsburg Subscriptions to the PENNSYLVANIA ANGLER EDGAR W. NICHOLSON should be addressed to the Editor. Submit fee either Philadelphia by check or money order payable to the Commonwealth of Pennsylvania. Stamps not acceptable. Individuals KENNETH A. REID sending cash do so at their own risk. Connellsville CHARLES A. FRENCH < Ellwood City HARRY E. WEBER PENNSYLVANIA ANGLER welcomes contributions Philipsburg and photos of catches from its readers. Proper credit will be given to contributors. MILTON L. PEEK All contributions returned if accompanied by first Ithan class postage. H. R. STACKHOUSE Secretary to Board ••» .,<>.. IMPORTANT—The Editor should be notified immediately of change in subscriber's address. Please give both old and new addresses Permission to reprint will be granted provided proper credit notice is given ^ANGLERT Vol.4 No. 11 NOVEMBER, 1935 EDITORIAL them do not know the essential dif Junior Sportsmen ferences in shape, coloration and It is my belief that the conserva build of our inland water fishes. Un tion movement, not only in Pennsyl fortunately, size limits also are too vania but in the United States, can scantily known. -

How the Scallop Shell Became the Emblem of the Methodist Church
How the Scallop Shell became the Emblem of the Methodist Church In 1778 the portrait painter William Hamilton RA There is evidence that Charles Wesley turned down an painted the portrait of John Wesley which now offer to inherit the Coat of Arms and a fortune belonging hangs in the National Portrait Gallery in London. to Garrett Wesley, this going eventually to the Duke of Wellington. Later that same year, an engraving of this portrait was published by James Fittler. Beneath the portrait, We should remember it is through Wesley's Coat of Arms Fittler added his own conception of the Coat of Arms that we are linked to the spirit of all those who joined in of the Wesley family – a shield with an outlined the “Crusade for Christ”. cross, containing three scallop shells in each quarter and a wyvern as the crest, with the words, “God is ___________________________________ love” as the motto underneath. It is not known whether he prepared this drawing with Wesley's permission, but the motto added an 1. The scallop shell comes from Wesley's authentic touch, for Wesley did use the words, “God Coat of Arms. is love” on one of his seals. ___________________________________ It seems that there are as many as 15 different Coat Designed by Ben Matthee for the of Arms used by various branches of the Wesley 2. The cross is central, reminding us of Methodist Centenary celebrated family, but the one under John Wesley's portrait has Christ’s one perfect and sufcient sacrice become a fairly well-known Methodist motif, even throughout South Africa in 1982, though it cannot strictly live up to its title of being the emblem has become very much for the world’s sins. -

THE ORIGINS of the “Mccrackens”
THE ORIGINS OF THE “McCrackens” By Philip D. Smith, Jr. PhD, FSTS, GTS, FSA Scot “B’e a’Ghaidhlig an canan na h’Albanaich” – “Gaelic was the language of the Scottish people.” The McCrackens are originally Scottish and speakers of the Scottish Gaelic language, a cousin to Irish Gaelic. While today, Gaelic is only spoken by a few thousands, it was the language of most of the people of the north and west of Scotland until after 1900. The McCracken history comes from a long tradition passed from generation to generation by the “seannachies”, the oral historians, of the Gaelic speaking peoples. According to tradition, the family is named for Nachten, Lord of Moray, a district in the northeast of Scotland. Nachten supposedly lived in the 9th century. In the course of time a number of his descendants moved southwest across Scotland and settled in Argyll. The family multiplied and prospered. The Gaelic word for “son” is “mac” and that for “children” is “clann” The descendants of Nachten were called by their neighbors, the Campbells, MacDougalls, and others the “Children of the Son of Nachten”, in Gaelic “Cloinne MacNachtain”, “Clan MacNachtan”. Spelling was not regularized in either Scotland or America until well after 1800. Two spellings alternate for the guttural /k/-like sound common in many Gaelic words, -ch and –gh. /ch/ is the most common Scottish spelling but the sound may be spelled –gh. The Scottish word for “lake” is “loch” while in Northern England and Ireland the same word is spelled “lough”. “MacLachlan” and “Mac Loughlin” are the same name as are “Docherty” and “Dougherty”. -

Heraldic Arms and Badges
the baronies of Duffus, Petty, Balvenie, Clan Heraldic Arms and Aberdour in the northeast of Murray Clan On 15 May 1990 the Court of Lord Scotland, as well as the lordships of Lyon granted The Murray Clan Society Bothwell and Drumsargard and a our armorial ensign or heraldic arms. An Society number of other baronies in lower armorial ensign is the design carried on Clydesdale. Sir Archibald, per the a flag or shield. English property law of jure uxoris, Latin for "by right of (his) wife" became the The Society arms are described on th th Clan Badges legal possessor of her lands. the 14 page of the 75 Volume of Our Public Register of All Arms and Bearings and Heraldic Which Crest Badge to Wear in Scotland, VIDELICT as: Azure, five Although Murrays were permitted to annulets conjoined in fess Argent wear either the mermaid or demi-man between three mullets of the Last. Above Arms crest badges, sometime in the late the Shield is placed an Helm suitable to Clan Badges 1960’s or early 1970’s, the Lord Lyon an incorporation (VIDELICET: a Sallet Prior to the advent of heraldry, King of Arms declared the demi-man Proper lined Scottish clansmen and clanswomen crest badge inappropriate. Since his Gules) with a wore badges to identify themselves. decisions on heraldic matters have the Clan badges were devices with family or force of law in Scotland, all the personal associations which identified manufacturers of clan badges, etc., the possessor, not unlike our modern ceased producing the demi-man. There class rings, military insignias, union pins, was a considerable amount of feeling on etc. -

Foglio E Bollettino Ufficiale N. 102/2017 Del 22.12.2017 Atti Legislativi E Dell'amministrazione Pagina 1 Di 7 Fogl
Foglio Ufficiale Pagina 1 di 7 Foglio e Bollettino ufficiale www.ti.ch/fu N. 102/2017 del 22.12.2017 Atti legislativi e dell'amministrazione Pubblicazione annuale dei periti e supplenti comunali degli immobili locativi per il periodo 1° gennaio 2018 - 31 dicembre 2018 N. Comune Perito Perito supplente 1 Acquarossa Saporito Monga Gelmino, Pasquale, Acquarossa Motto Blenio 2 Agno Pellegatta Giulio, Da designare Agno 3 Airolo Thomas Marco, Gianola Cristian, Airolo Airolo 4 Alto Malcantone Devittori Rella Matteo, Beatrice, Vezio Gravesano 5 Aranno Collura Massimo, Vanetta Paolo, Vezia Morbio Inferiore 6 Arbedo-Castione Belotti Enrico, Rè Guido, Arbedo Arbedo 7 Arogno Vicari Claudio, Bianchi Mauro, Arogno Stabio 8 Ascona Masoni Jasmine, Cerutti Paola, Verscio Ascona 9 Astano Wyrsch Peter, Hess Claudio, Astano Astano 10 Avegno Gordevio Dalessi Andrea, Felder Giorgio, Cavergno Maggia 11 Balerna Canonico Valeria, Ballabio Brunello, Balerna Vacallo 12 Bedano Petrillo Vito, Codoni Gottardo, Sigirino Bedano 13 Bedigliora Custer Lorenzo, Marcoli Fausto, Beride Novaggio 14 Bedretto Pedrini Andrea, Sambusiti Gianni, Faido Chironico 15 Bellinzona Germann Scapozza Nello, Giacomo, Bellinzona Bellinzona Comprensori di Giubiasco, Bruschi Pallua Marco, Pianezzo Alessandro, Sementina e St. Antonio Mendrisio Comprensorio De Luigi Ivano, di Camorino Camorino https://www3.ti.ch/CAN/FoglioUfficiale/public/index.php/ricerca/risultato/ 19. 11. 2018 Foglio Ufficiale Pagina 2 di 7 Pedrioli Emanuele, Gorduno Comprensorio Imperatori Luca, Del Don Angelo, di Claro Bellinzona -
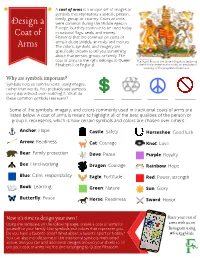
Design a Coat of Arms
A coat of arms is a unique set of images or symbols that represents a specific person, family, group, or country. Coats of arms Design a were common during the Middle Ages in Europe, but they continue to be used today in national flags, seals, and money. Coat of Elements that are common on coats of arms include shields, animals, and mottos. Arms The colors, symbols, and imagery are specifically chosen to tell you something about that person, group, or family. The coat of arms to the right belongs to Queen The Royal Arms of the United Kingdom, featuring Elizabeth II of England. a shield in the center and a motto on the bottom. Courtesy of Encyclopædia Britannica. Why are symbols important? Symbols help us communicate using images, rather than words. You probably see symbols every day without even realizing it. What do these common symbols represent? Some of the symbols, imagery, and colors commonly used in traditional coats of arms are listed below. A coat of arms is meant to highlight all of the best qualities of the person or group it represents, which is how certain symbols and colors are chosen over others. Anchor: Hope Castle: Safety Horseshoe: Good luck Arrow: Readiness Cat: Courage Knot: Love Bear: Family protection Dove: Peace Purple: Royalty Bee: Hard-working Dragon: Courage Rainbow: Hope Blue: Calm, responsibility Eagle: Fortitude Red: Power, strength Book: Learning Green: Nature Sun: Glory Butterfly: Peace Horse: Readiness Sword: Honor Now it's time to design your own! Share your coat of Using the template on the following page, create a coat of arms for arms with us on yourself or your family. -

Archbishop Carlson
Most Rev. Carlson's Coat of Arms Motto: ANTE CRUCEM NIHIL DEFENSIONIS | Before the Cross there is no Defense The arms of a diocesan bishop are displayed on a heraldic shield divided vertically into two portions. The arms of the Catholic Diocese of Saginaw are the dexter impalement shown on the half of the shield to the viewer's left. The arms of the Bishop Carlson are on the sinister impalement, to the viewer's right. The personal arms of Bishop Carlson were designed by the Rev. James Parker. The "garb" is the heraldic representation of the sheaf of wheat and represents the bishop's native city of Minneapolis, Minn., known as the "Miller of the Midwest". The field of green suggests the great plains of South Dakota as well as the liturgical color for growth in the Faith. On the field, on either side of the sheaf of wheat are a crescent and a Latin Cross. The silver crescent represents Mary Immaculate and the Cross represents the bishop's faith committment and his mission to the faithful entrusted to his care. The heraldic cheif at the top of the shield is taken from the family arms of the bishop. The wavy bars in the base of the shield represent the Sioux River and the bishop's service in the Diocese of Sioux Falls. The scroll, bearing the bishop's motto is placed below the shield. As his watchword, the bishop chose: ANTE CRUCEM NIHIL DEFENSIONIS, which means "Before the Cross there is no defense". The entire achievement is completed by the addition of a gold processional cross that extends above and below the shield and a green ecclesiastical hat, the galero, with six tassles on each side disposed in three rows on either side of the shield. -

WHO DARES, WINS: the FUNCTION and EVOLUTION of PREDATOR INSPECTION BEHAVIOUR in SHOALING FISH by TONY PITCHER (Renewable Resourc
WHO DARES, WINS: THE FUNCTION AND EVOLUTION OF PREDATOR INSPECTION BEHAVIOUR IN SHOALING FISH by TONY PITCHER (RenewableResources Assessment Group, Imperial College, Londen, UK*) ABSTRACT This paper examines the experimental evidence which underpins our current under- standing of inspection behaviour in fish shoals, and reviews ideas about how predator inspection behaviour could have evolved. For fish which are vulnerable to attack in the presence of a predator, inspection is critical in the recognition of danger and in the precise assessment of risk on a second-to-second time scale. Up to a threshold, inspec- tion rates increase with danger, partly because new fish in the shoal begin to inspect. Inspection appears to have a genetic basis, although experience with predators during development can fine-tune it. Inspection is evidently a risky business, as shown indirectly by attack-cone avoidance and directly by predator strikes in experiments. An appraisal of the current state of a predator is transferred from inspectors to other individuals in the group: we do not know whether such information transfer is active or passive, or whether it involves an element of manipulation. In multispecies groups, some species seem to benefit from the inspection of others. There are large individual differences in inspection, so groups probably comprise two types of individuals with differing trade-offs between risk and information. A key question is how such apparently altruistic behaviour can have evolved. Among putative functions which have been examined, inspection seems to facilitate anticipa- tion of attack, but hypotheses of attack inhibition, attack invitation, and approach conditional on danger have not been supported unequivocally by experimental results. -

Recipe Ideas for Farmed Sea Scallops the Whole Story
Recipe Ideas for Farmed Sea Scallops The Whole Story By Marsden Brewer & Marnie Reed Crowell Foreword by Master Chef Barton Seaver Recipe Ideas for Farmed Sea Scallops The Whole Story By Marsden Brewer & Marnie Reed Crowell Foreword by Master Chef Barton Seaver Recipie Ideas for Farmed Sea Scallops: The Whole Story Copyright © 2020 Marsden Brewer ISBN: 978-0-9802177-8-0 All rights reserved. No part of this book may be reproduced in any form or by any electronic or mechanical means, including information storage and retrieval systems, without permission in writing from the author, except by a reviewer, who may quote brief passages in review. Printed in the United States of America Table of Contents Foreword ..................................................................................................1 Handling Scallops ...........................................................................5 Petites—The Smallest ................................................................11 Butter-Braised PenBay “Popcorn” ....................................... 13 Steam shucking .............................................................................. 14 Amuse-bouche ..................................................................................15 Medium Size ..........................................................................................17 Spain ............................................................................................... 19 Manchego Scallops .................................................................. -

St. John's, Newfoundland and Labrador
St. John’s, Newfoundland and Labrador 185 St. John’s, Newfoundland and Labrador ✪ Population Rank: Canada. 20 Province. .1 Proportions: 1:2 Adopted: Unknown (arms granted 1965) DESIGN: The flag of the City of St. John’s has a white field with its colour- ful coat of arms, nearly the full height of the flag, in the centre. The simple shield has a horizontal top and simply-curved sides forming a pointed “U” shape. It has a red field, with a white section on its top third bearing three wavy blue stripes with three undulations at its base. Atop the wavy stripes is an early ship sailing toward the hoist with a jib and square main sail in white and a golden yellow hull. A long pennant blows forward from its single mast, a smaller ensign from a staff in the stern, both in red. In the centre of the lower section is a lamb oriented toward the hoist, depicted in white with black details, a golden yellow halo, and holding with its right foreleg a staff (ending in a cross finial) from which streams a swallow-tailed white flag bearing a red cross. On either side of the lamb is an inverted scallop shell in white outlined in black. Above the shield is a knight’s helmet in grey, white, and black with an elaborate crest: a golden yellow crown in the form of a crenulated stone wall with five towers, surrounding a rocky hill. On it stands a lion in golden yellow, with right foreleg raised between two red and white Tudor roses with green leaves and stems. -

Heraldry in Ireland
Heraldry in Ireland Celebrating 75 years of the Office of the Chief Herald at the NLI Sir John Ainsworth Shield Vert, a chevron between three battle-axes argent Crest A falcon rising proper, beaked, legged and belled gules Motto Surgo et resurgam Did you know? Sir John Ainsworth was the NLI's Surveyor of Records in Private Keeping in the 1940s and 1950s. Roderick More OFerrall Shield Quarterly: 1st, Vert, a lion rampant or (for O Ferrall); 2nd, Vert a lion rampant in chief three estoiles or (for O More); 3rd, Argent, upon a mount vert two lions rampant combatant gules supporting the trunk of an oak tree entwined with a serpent descending proper, (for O Reilly); 4th, Azure, a bend cotised or between six escallops argent (for Cruise) Crest On a ducal coronet or a greyhound springing sable; A dexter hand lying fess-ways proper cuffed or holding a sword in pale hilted of the second pierced through three gory heads of the first Motto Cú re bu; Spes mea Deus Did you know? This four designs on the shield represent four families. Heiress Leticia More of Balyna, county Kildare married Richard Ferrall in 1751. Their grandson Charles Edward More O'Ferrall married Susan O'Reilly in 1849. Susan was the daughter of Dominic O'Reilly of Kildangan Castle, county Kildare who had married heiress Susanna Cruise in 1818. Dublin Stock Exchange Shield Quarterly: 1st, Sable, a tower or; 2nd, Vert, three swords points upwards two and one proper pommelled and hilted or; 3rd, Vert, three anchors erect two and one argent; 4th, Chequy, sable and argent, on a chief argent an escroll proper, inscribed thereon the words Geo. -

Rapporto Finale Della Commissione Di Studio Sull'aggregazione Del Bellinzonese
RAPPORTO FINALE DELLA COMMISSIONE DI STUDIO Bellinzona, 26 marzo 2015 Progetto aggregativo dei Comuni dell’agglomerato del Bellinzonese Comuni di Arbedo-Castione, Bellinzona, Cadenazzo, Camorino, Claro, Giubiasco, Gnosca, Gorduno, Gudo, Lumino, Moleno, Monte Carasso, Pianezzo, Preonzo, S. Antonino, S. Antonio, Sementina Rapporto finale della Commissione di studio Sommario Sintesi ...............................................................................................................................................................10 PARTE A 1 Organizzazione del progetto .....................................................................................................................21 2 Obiettivi del rapporto ................................................................................................................................21 3 La strategia cantonale delle aggregazioni ................................................................................................22 4 Il contesto dell’agglomerato di Bellinzona e la sua evoluzione recente ....................................................26 PARTE B 5 Una nuova Città: perché ? ........................................................................................................................29 5.1 Carta dei valori ............................................................................................................................29 5.2 Visione .........................................................................................................................................29