Dendrobiumflower Color
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Diversity of Wild Orchids in the Southern Slope of Mount Merapi, Yogyakarta, Indonesia Eight Years After the 2010 Eruption
BIODIVERSITAS ISSN: 1412-033X Volume 21, Number 9, September 2020 E-ISSN: 2085-4722 Pages: 4457-4465 DOI: 10.13057/biodiv/d210964 The diversity of wild orchids in the southern slope of Mount Merapi, Yogyakarta, Indonesia eight years after the 2010 eruption FEBRI YUDA KURNIAWAN1,2,♥, FAUZANA PUTRI2,3, AHMAD SUYOKO2,3, HIMAWAN MASYHURI2,3, MAYA PURQI SULISTIANINGRUM2,3, ENDANG SEMIARTI3,♥♥ 1Postgraduate School, Universitas Gadjah Mada. Jl. Teknika Utara, Sleman 55281, Yogyakarta, Indonesia. Tel./fax. +62-274-544975, email: [email protected] 2Biology Orchid Study Club (BiOSC), Faculty of Biology, Universitas Gadjah Mada. Jl. Teknika Selatan, Sekip Utara, Sleman 55281, Yogyakarta, Indonesia 3Department of Tropical Biology, Faculty of Biology, Universitas Gadjah Mada. Jl. Teknika Selatan, Sekip Utara, Sleman 55281, Yogyakarta, Indonesia. Tel./fax.: +62-274-580839, email: [email protected] Manuscript received: 21 August 2020. Revision accepted: 31 August 2020. Abstract. Kurniawan FY, Putri F, Suyoko A, Masyhuri H, Sulistianingrum MP, Semiarti E. 2020. The diversity of wild orchids in the southern slope of Mount Merapi, Yogyakarta, Indonesia eight years after the 2010 eruption. Biodiversitas 21: 4457-4465. The ecosystem of the slopes of Mount Merapi is mountain tropical forest which is frequently affected by volcanic activities. The dynamics of the volcano affect the diversity and abundance of orchids in the ecosystem. Tritis is an area included in the Turgo Hill of the southern slope of Mount Merapi and is under the management of Mount Merapi National Park. The ecosystem in Tritis area classified as lower mountain forest and it has been affected by Mount Merapi eruption. This study aimed to do an inventory of orchid species in Tritis to know the diversity and abundance of orchids that exist in this area. -

Vascular Epiphytic Medicinal Plants As Sources of Therapeutic Agents: Their Ethnopharmacological Uses, Chemical Composition, and Biological Activities
biomolecules Review Vascular Epiphytic Medicinal Plants as Sources of Therapeutic Agents: Their Ethnopharmacological Uses, Chemical Composition, and Biological Activities Ari Satia Nugraha 1,* , Bawon Triatmoko 1 , Phurpa Wangchuk 2 and Paul A. Keller 3,* 1 Drug Utilisation and Discovery Research Group, Faculty of Pharmacy, University of Jember, Jember, Jawa Timur 68121, Indonesia; [email protected] 2 Centre for Biodiscovery and Molecular Development of Therapeutics, Australian Institute of Tropical Health and Medicine, James Cook University, Cairns, QLD 4878, Australia; [email protected] 3 School of Chemistry and Molecular Bioscience and Molecular Horizons, University of Wollongong, and Illawarra Health & Medical Research Institute, Wollongong, NSW 2522 Australia * Correspondence: [email protected] (A.S.N.); [email protected] (P.A.K.); Tel.: +62-3-3132-4736 (A.S.N.); +61-2-4221-4692 (P.A.K.) Received: 17 December 2019; Accepted: 21 January 2020; Published: 24 January 2020 Abstract: This is an extensive review on epiphytic plants that have been used traditionally as medicines. It provides information on 185 epiphytes and their traditional medicinal uses, regions where Indigenous people use the plants, parts of the plants used as medicines and their preparation, and their reported phytochemical properties and pharmacological properties aligned with their traditional uses. These epiphytic medicinal plants are able to produce a range of secondary metabolites, including alkaloids, and a total of 842 phytochemicals have been identified to date. As many as 71 epiphytic medicinal plants were studied for their biological activities, showing promising pharmacological activities, including as anti-inflammatory, antimicrobial, and anticancer agents. There are several species that were not investigated for their activities and are worthy of exploration. -

A Review of CITES Appendices I and II Plant Species from Lao PDR
A Review of CITES Appendices I and II Plant Species From Lao PDR A report for IUCN Lao PDR by Philip Thomas, Mark Newman Bouakhaykhone Svengsuksa & Sounthone Ketphanh June 2006 A Review of CITES Appendices I and II Plant Species From Lao PDR A report for IUCN Lao PDR by Philip Thomas1 Dr Mark Newman1 Dr Bouakhaykhone Svengsuksa2 Mr Sounthone Ketphanh3 1 Royal Botanic Garden Edinburgh 2 National University of Lao PDR 3 Forest Research Center, National Agriculture and Forestry Research Institute, Lao PDR Supported by Darwin Initiative for the Survival of the Species Project 163-13-007 Cover illustration: Orchids and Cycads for sale near Gnommalat, Khammouane Province, Lao PDR, May 2006 (photo courtesy of Darwin Initiative) CONTENTS Contents Acronyms and Abbreviations used in this report Acknowledgements Summary _________________________________________________________________________ 1 Convention on International Trade in Endangered Species (CITES) - background ____________________________________________________________________ 1 Lao PDR and CITES ____________________________________________________________ 1 Review of Plant Species Listed Under CITES Appendix I and II ____________ 1 Results of the Review_______________________________________________________ 1 Comments _____________________________________________________________________ 3 1. CITES Listed Plants in Lao PDR ______________________________________________ 5 1.1 An Introduction to CITES and Appendices I, II and III_________________ 5 1.2 Current State of Knowledge of the -

Synthetic Seeds of Endangered Medicinal Orchid Species, Dendrobium Crumenatum Sw
Volume 6 Number 1, January 2018 Synthetic Seeds of Endangered Medicinal Orchid Species, Dendrobium crumenatum Sw. Sutha Klaocheed1and Suphat Rittirat2 1Faculty of Science and Technology, Prince of Songkla University, Pattani campus, 2Faculty of Science and Technology, Nakhon Si Thammarat Rajabhat University, Thailand. ABSTRACT exchanges among countries in the floriculture trade. In this study, this Climate change and anthropogenic pressure severely method was used to study the bead threaten plant genetic diversity formations and the conversion worldwide. Numerous species are capabilities of D. crumenatum Sw. For synthetic seed, the superior gel matrix described as rare or endangered, and integrated programs are required to for encapsulation of D. crumenatum protect and preserve current Sw. was obtained using 3 % (w/v) biodiversity. Ex situ conservation sodium-alginate and 100 mM calcium methods played an important role in chloride for 30 minutes. Successful storage of capsules, until 105 days, the conservation of threatened plants. o The main methods used in ex situ was achieved at 8 ± 2 C with conservation are maintenance of conversion frequency of 50.0 % when living plants through cultivation, in culture on MS medium supplemented vitro conservation and encapsulation. with 0.2 % (w/v) activated charcoal An in vitro plant regeneration protocol (AC). Well-rooted plantlets derived was successfully established for from capsules were acclimatized in threatened medicinal species, the greenhouse with 95 % survival Dendrobium crumenatum Sw. by rate. The regeneration protocol culturing axillary buds. Protocorm- developed in this study provides a like bodies (PLBs) of D. crumenatum basis for ex-situ germplasm Sw. can be induced from callus conservation of medicinal importance segments cultured on MS (Murashige present in D. -

Malaysian Limestone Orchids Status: Diversity, Threat and Conservation
Blumea 54, 2009: 109–116 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X474168 Malaysian limestone orchids status: diversity, threat and conservation G. Rusea1, M.Y.L. Lim1, S.N. Phoon2, W.S.Y. Yong2, C.H. Tang1, H.E. Khor1, J.O. Abdullah1, J. Abdullah3 Key words Abstract To date, a total of 288 species from 96 genera were identified from the limestone areas in Perlis and Padawan-Bau, Sarawak, of which many of these are restricted to limestone habitat and either endemic to Perlis or conservation to Sarawak. Knowledge and data obtained from the field observation over the past 8 years leads us to report that diversity at least 15 species endemic to limestone has become rare in the wild in Perlis, Bau and Padawan Sarawak. This limestone orchids was mainly attributed by: i) lack of emphasis by the government on understanding and protecting biodiversity in Malaysian this kind of habitat; ii) lack of scientists willing to do research in dangerous and disaster prone limestone habitat; threat and iii) lack of knowledge and awareness among local communities on the importance of conserving and utilizing their natural resources in a sustainable manner. Published on 30 October 2009 INTRODUCTION Material AND METHODS Orchids are the largest flowering plant family in Malaysia In both areas limestone hills and some adjacent landscape (including Sabah and Sarawak) with about 2 000 species, of features were selected for this survey (Table 1). In Sarawak, two which 700 are recorded from limestone. Threats to orchids on rivers were included that flow through the limestone hills and limestone include small-scale logging (extracting timber by valleys. -

PHES12 343-403.Pdf
343 Insects Associated with Orchids By O. H. SWEZEY Consulting Entomologist Experiment Station, H.S.P.A., Honolulu CONTENTS PAGE PAGE Introduction --- ••— 344 Heteroptera " - ----- 367 Coleoptera apparently attached Miridae (Plant bugs attached- . to orchids) 367 to orchids Curculionidae :: 345 Miscellaneous bugs intercepted Orchid weevils in Hawaii.... 345 on orchids 368 Orchid weevils known else Cydnidae 368 where than in Hawaii 349 Pentatomidae 3t>9 Scolytidae 352 Coreidae 369 Mordellistenidae 3W Lygaeidae - --- 370 Cerambycidae 354 Pyrrhocondae o/i Hispidae 354 Tingitidae 371 Chrysomelidae 355 - Aradidae : 3J2 List of Intercepted beetles 355 Miridae - -372 Chrysomelidae 355 Homoptera *. *'* Tenebrionidae 356 Aphididae - 372 Aleurodidae' .: * 3/6 Cucujidae - - - 357 Psyllidae 374 Trixagidae - M ' Lampyridae &» Membracidae - ^ Elateridae - - ^/ Coccidae r -;—- 3/4 List of scale insects for which Dermestidae 358 Lyctidae 358 orchids are the sole or Colidiidae 358 chief food plant 374 Anthribidae 358 List of scale insects having diverse food plants, in Hydrophilidae —• 358 cluding orchids -- 382 Scaphydiidae 358 Ptinidae 358 Orthoptera - -- 390 Melandryidae ^° Tettigoniidae ^ Coccinellidae - 358 Locustidae 392 Scarabaeidae ......— - 359 Gryllidae '- : 392 Endomychidae -• 359 Phasmidae I - 392 Scolytidae 359 Dermaptera - 392 Hymenoptera • 359 Roaches - —- 393 Eurytomidae - 6^/ Thysanoptera 393 Xylocopidae 360 Thrips described from orchids.. 393 Formicidae - ^ Thrips incidentally on orchids Lepidoptera - 362 or intercepted on imported Lycaenidae &£ orchids - 395 Castniidae • 362 Embioptera - - 396 Geometridae 364 Limacodidae ^4 Isoptera 397 Lithosiadae 364 Collembola - - 397 Liparidae 365 Insects which pollinate orchids.. 397 Plusiadae ; 365 Butterflies 398 Psychidae - 3o5 Moths 398 Pyralidae - 365 Bees 399 Tortricidae ^ Stinging ants y\ Cosmopterygidae 366 Wasps - ■■■■■ 401 Acrolophidae Flies ■ ■ 401 366 Diptera Diptera (undetermined) 402 Cecidomyiidae 366 Beetles - - 402 Tephritidae - 367 Thrips 402 Anthomyiidae 60/ Proc. -

An Assessment of Orchids' Diversity in Penang Hill, Penang, Malaysia After
Biodivers Conserv (2011) 20:2263–2272 DOI 10.1007/s10531-011-0087-z ORIGINAL PAPER An assessment of orchids’ diversity in Penang Hill, Penang, Malaysia after 115 years Rusea Go • Khor Hong Eng • Muskhazli Mustafa • Janna Ong Abdullah • Ahmad Ainuddin Naruddin • Nam Sook Lee • Chang Shook Lee • Sang Mi Eum • Kwang-Woo Park • Kyung Choi Received: 22 September 2010 / Accepted: 3 June 2011 / Published online: 12 June 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract A comprehensive study on the orchid diversity in Penang Hill, Penang, Malaysia was conducted from 2004 to 2008 with the objective to evaluate the presence of orchid species listed by Curtis (J Strait Br R Asiat Soc 25:67–173, 1894) after more than 100 years. A total of 85 species were identified during this study, of which 52 are epiphytic or lithophytic and 33 are terrestrial orchids. This study identified 57 species or 64.8% were the same as those recorded by Curtis (1894), and 78 species or 66.1% of Turner’s (Gar- dens’ Bull Singap 47(2):599–620, 1995) checklist of 118 species for the state of Penang including 18 species which were not recorded by Curtis (1894) and the current study but are actually collected from Penang Hill. A comparison table of the current findings against Curtis (1894) and Turner (1995) is provided which shows only 56 species were the same in all three studies. The preferred account for comparison was Curtis’ (1894) list as his report was specifically for the areas around Penang Island especially Penang Hill, Georgetown and Ayer Itam areas. -

Phylogenetic Relationships of Selected Sri Lankan Orchids Based on Internal Transcribed Spacer (ITS) Sequence Analysis
ISSN (Online): 2349 -1183; ISSN (Print): 2349 -9265 TROPICAL PLANT RESEARCH 7(1): 76–85, 2020 The Journal of the Society for Tropical Plant Research DOI: 10.22271/tpr.2020.v7.i1.011 Research article Phylogenetic relationships of selected Sri Lankan Orchids based on Internal Transcribed Spacer (ITS) sequence analysis P. M. H. Sandamali1, 4, S. P. Senanayake2* and Sanath Rajapakse3, 4 1Floriculture Research Center, University of Kelaniya, Kelaniya, Sri Lanka 2Department of Plant and Molecular Biology, University of Kelaniya, Kelaniya, Sri Lanka 3Department of Molecular Biology and Biotechnology, University of Peradeniya, Peradeniya, Sri Lanka 4 Postgraduate Institute of Science, University of Peradeniya, Peradeniya, Sri Lanka *Corresponding Author: [email protected] [Accepted: 07 March 2020] Abstract: Orchidaceae is a widespread plant family and Sri Lankan orchid flora represents 188 species belonging to 78 genera, including 01 endemic genus (Adrorhizon) and 55 endemic species. The main aim of the present research was to characterize selected species of genera Dendrobium and Bulbophyllum in Sri Lanka with respect to their ITS sequence data and to derive phylogenetic relationships. Modified CTAB protocol was followed for DNA extractions and ITS region was amplified using the primer sets of 17SE and 26SE and phylogenetic trees were constructed based on the available ITS sequences of the Asian species of Dendrobium and Bulbophyllum by MEGA7 software package. Genetic variation and relationships of six Sri Lankan orchid species; Dendrobium aphyllum, D. crumenatum, D. nutantiflorum endemic species of Bulbophyllum elliae, B. trimenii and Eria bicolor were determined using ITS sequencing. Findings of the analysis conclude, suitability of ITS sequencing as a molecular marker for deriving phylogenetic relationships of genera Dendrobium and Bulbophyllum with Eria as the out group. -
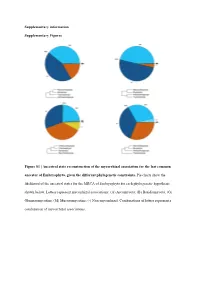
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

IDENTIFICATION and MODE of SYMBIOSIS for MYCORRHIZAL FUNGI and EPIPHYTIC ORCHID, Dendrobium Crumenatum
IDENTIFICATION AND MODE OF SYMBIOSIS FOR MYCORRHIZAL FUNGI AND EPIPHYTIC ORCHID, Dendrobium crumenatum JALILAH BINTI RAPIE UNIVERSITI SAINS MALAYSIA 2016 IDENTIFICATION AND MODE OF SYMBIOSIS FOR MYCORRHIZAL FUNGI AND EPIPHYTIC ORCHID, Dendrobium crumenatum by JALILAH BINTI RAPIE Thesis submitted in fulfillment of the requirements for the degree of Master of Sciences March 2016 ACKNOWLEDGEMENT In the name of Allah S.W.T, The Most Gracious, The Most Merciful Assalamualaikum W.B.T, Alhamdulillah, On behalf of myself, Jalilah binti Rapie, I want to express my thanks to Allah S.W.T in the first place for giving me the will and the strength to face the hardship upon the completion of this Master’s project. There are numbers of people that I would like to thank for their contributions to the completion of this final year project. First expression of my gratitude’s and my highest appreciation is to my supervisor Assc. Prof. Dr. Hideyuki Dhaakirullah Nagao for his constructive criticisms, suggestions, guidance and opinions in doing and finishing this Master’s project. Throughout conducting this project, he gave me a lot of new knowledge and experience. Special thanks are owed to my friends, whom are under the same supervisor and did their Master’s project in the same laboratory. Without their co-operation and help, I would not have been able to do Master’s project completely. To all staffs of School of Biological Sciences, I sincerely thank all of you for showing the chores and other things that related to my project. Last but not least, my greatest appreciation is owed to my parents Encik Rapie bin Famri and Puan Fatimah binti Abas for giving me the encouragement and support during doing and finishing this Master’s project. -

Download Download
BIODIVERSITAS ISSN: 1412-033X Volume 21, Number 5, May 2020 E-ISSN: 2085-4722 Pages: 2244-2250 DOI: 10.13057/biodiv/d210554 Orchid exploration in Tanjung Peropa Wildlife Reserves for Kendari Botanic Gardens collection, Indonesia SRI HARTINI, POPI APRILIANTI Research Center for Plant Conservation and Botanic Gardens, Indonesian Institute of Sciences. Jl. Ir. H. Juanda 13, Bogor 16122, West Java, Indonesia. Tel./fax.: +62-251-8322187, email: [email protected] Manuscript received: 14 January 2019. Revision accepted: 27 April 2020. Abstract. Hartini S, Aprilianti P. 2020. Orchid exploration in Tanjung Peropa Wildlife Reserves for Kendari Botanic Gardens collection, Indonesia. Biodiversitas 21: 2244-2250. Tanjung Peropa Wildlife Reserve (Tanjung Peropa WR) is one of the conservation area in Southeast Sulawesi Province, Indonesia. The inventory of orchid in this area is still limited and Kendari Botanic Garden (Kendari BG) need to collect the orchids from this location. Orchid diversity inventory and exploration had been carried out on March 26- April 12, 2019, in Tanjung Peropa WR and collecting them as an ex-situ conservation effort in order to enrich Kendari BG plant collection. The specimen was collected by using explorative method at 5 locations in Tanjung Peropa WR. The result showed that there were 10 epiphytic orchids found of Aerides, Cymbidium, Dendrobium, Grammatophyllum, Liparis, Pomatocalpa, and Thrixspermum. Also, there were 5 terrestrial orchids of Corymborkis, Eulophia, Nervilia, Phaius, and Tropidia. The orchid species were found in the area between 25-110 m above sea level (asl), with temperature 27-31°C, humidity 75-80%, soil acidity 5.0-6.0, soil humidity 80-90%, and canopy dense between 60-80%. -

Present and Future Distribution of Two Non-Indigenous Orchids and Their Acquired Enemy in Puerto Rico
Present and Future Distribution of Two Non-Indigenous Orchids and Their Acquired Enemy in Puerto Rico Evan A. Foster ( [email protected] ) Colorado College https://orcid.org/0000-0002-1311-378X James Ackerman University of Puerto Rico Rio Piedras: Universidad de Puerto Rico Recinto de Rio Piedras Research Article Keywords: Maxent, Orchidaceae, herbivory, climate change, biotic resistance, island invasion Posted Date: February 23rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-220086/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Biological Invasions on July 12th, 2021. See the published version at https://doi.org/10.1007/s10530-021-02596-3. Page 1/18 Abstract Establishment of new populations is contingent on overcoming abiotic and biotic barriers. While this applies to all species, these hurdles are at the forefront of invasion biology where prediction, prevention, eradication, and control strategies depend on an understanding of these processes. Arundina graminifolia and Dendrobium crumenatum are two non-indigenous orchids spreading throughout Puerto Rico. The two species have acquired a native herbivore & seed predator, the orchid-specialist weevil, Stethobaris polita. With recently acquired presence records of the three species, land cover data and BioClim variables, we modeled their potential distributions under current conditions and also those projected under the least and most extreme climate scenarios for 2050 and 2070. We show that D. crumenatum ourishes in urban environments which also provide refugia from S. polita, whereas there is currently limited refugia for A.