RECAUSTICIZING - PRINCIPLES and Figure 1- a Simple Diagram of the Recausticizing Flowsheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Master's Thesis
2008:081 MASTER'S THESIS Pulping Wastes and Abandoned Mine Remediation Application of green liquor dregs and other pulping by-products to the solidification/stabilisation of copper mine tailings Lucile Villain Luleå University of Technology D Master thesis Chemistry Department of Civil and Environmental Engineering Division of Architecture and Infrastructure 2008:081 - ISSN: 1402-1552 - ISRN: LTU-DUPP--08/081--SE Luleå University of Technology MASTER THESIS Pulping Wastes and Abandoned Mine Remediation Application of green liquor dregs and other pulping by-products to the solidification/stabilisation of copper mine tailings Lucile Villain June 2008 Department of Civil, Mining and Environmental Engineering Division of Architecture and Infrastructure ACKNOWLEDGEMENTS This master thesis was realised in Luleå University of Technology and in Ramböll Sverige AB consultancy in Luleå (Northern Sweden). I would like to express my gratitude to my supervisor Christian Maurice who made the project possible and who guided me throughout this work while granting me autonomy as well. I am also grateful to Ramböll team who nicely welcomed me in spite of my poor Swedish, and helped in practical issues. I would like to thank Nils Hoffner for his useful information, Tomas Forsberg for his valuable help in the laboratory, Ulla-Britt Uvemo for her kind and constant assistance, and Lea Rastas Amofah for her friendly company and wise advice. I am also thankful towards my family who encouraged me and sent me motivation from France; many thanks to my friends in Luleå who gave me support and joy during these days. 1 ABSTRACT Green liquor dregs are one type of chemical by-products produced by the pulp and paper industry which are usually landfilled, and cause concern to the pulp mills due to the cost of landfilling. -
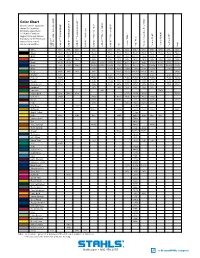
Color Chart ® ® ® ® Closest Pantone® Equivalent Shown
™ ™ II ® Color Chart ® ® ® ® Closest Pantone® equivalent shown. Due to printing limitations, colors shown 5807 Reflective ® ® ™ ® ® and Pantone numbers ® ™ suggested may vary from ac- ECONOPRINT GORILLA GRIP Fashion-REFLECT Reflective Thermo-FILM Thermo-FLOCK Thermo-GRIP ® ® ® ® ® ® ® tual colors. For the truest color ® representation, request Scotchlite our material swatches. ™ CAD-CUT 3M CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT Felt Perma-TWILL Poly-TWILL Thermo-FILM Thermo-FLOCK Thermo-GRIP Vinyl Pressure Sensitive Poly-TWILL Sensitive Pressure CAD-CUT White White White White White White White White White* White White White White White Black Black Black Black Black Black Black Black Black* Black Black Black Black Black Gold 1235C 136C 137C 137C 123U 715C 1375C* 715C 137C 137C 116U Red 200C 200C 703C 186C 186C 201C 201C 201C* 201C 186C 186C 186C 200C Royal 295M 294M 7686C 2747C 7686C 280C 294C 294C* 294C 7686C 2758C 7686C 654C Navy 296C 2965C 7546C 5395M 5255C 5395M 276C 532C 532C* 532C 5395M 5255C 5395M 5395C Cool Gray Warm Gray Gray 7U 7539C 7539C 415U 7538C 7538C* 7538C 7539C 7539C 2C Kelly 3415C 341C 340C 349C 7733C 7733C 7733C* 7733C 349C 3415C Orange 179C 1595U 172C 172C 7597C 7597C 7597C* 7597C 172C 172C 173C Maroon 7645C 7645C 7645C Black 5C 7645C 7645C* 7645C 7645C 7645C 7449C Purple 2766C 7671C 7671C 669C 7680C 7680C* 7680C 7671C 7671C 2758U Dark Green 553C 553C 553C 447C 567C 567C* 567C 553C 553C 553C Cardinal 201C 188C 195C 195C* 195C 201C Emerald 348 7727C Vegas Gold 616C 7502U 872C 4515C 4515C 4515C 7553U Columbia 7682C 7682C 7459U 7462U 7462U* 7462U 7682C Brown Black 4C 4675C 412C 412C Black 4C 412U Pink 203C 5025C 5025C 5025C 203C Mid Blue 2747U 2945U Old Gold 1395C 7511C 7557C 7557C 1395C 126C Bright Yellow P 4-8C Maize 109C 130C 115U 7408C 7406C* 7406C 115U 137C Canyon Gold 7569C Tan 465U Texas Orange 7586C 7586C 7586C Tenn. -

Separation and Utilization of Solid Residue Streams from Kraft Pulp Mills
Separation and Utilization of Solid Residue Streams from Kraft Pulp Mills Master’s thesis in Innovative and Sustainable Chemical Engineering CECILIA STEEN Department of Chemistry and Chemical Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2016 Master’s thesis 2016 Separation and Utilization of Solid Residue Streams from Kraft Pulp Mills CECILIA STEEN Department of Chemistry and Chemical Engineering Division of Forest Products and Chemical Engineering Chalmers University of Technology Gothenburg, Sweden 2016 Separation and Utilization of Solid Residue Streams from Kraft Pulp Mills CECILIA STEEN © CECILIA STEEN, 2016. Supervisors: Tuve Mattsson, Department of Chemistry and Chemical Engineering Maria Björk, Stora Enso Biomaterials Rickard Wadsborn, Stora Enso Pulp Competence Centre Examiner: Hans Theliander, Department of Chemistry and Chemical Engineering Master’s Thesis 2016 Department of Chemistry and Chemical Engineering Division of Forest Products and Chemical Engineering Chalmers University of Technology SE-412 96 Gothenburg Telephone +46 31 772 1000 Cover: A schematic of the chemical recovery process of a Kraft pulp mill. Typeset in LATEX Printed by Department of Chemistry and Chemical Engineering Gothenburg, Sweden 2016 iv Separation and Utilization of Solid Residues Streams from Kraft Pulp Mills CECILIA STEEN Department of Chemistry and Chemical Engineering Chalmers University of Technology Abstract Separation of solid residues serve as an important kidney of non-process elements in a kraft pulp mill. The separation and later utilization of these residues as a possible source of nutrients in the forest is of interest in this thesis. One such solid residue is green liquor dregs, which are removed from the green liquor by means of filtration. -

Basics of Kraft Pulping
Lignin Wood is composed of many chemical components, primarily extractives, carbohydrates, and lignin, which are distributed nonuniformly as the result of anatomical structure. Lignin is derived from the Latin term lignum, which means wood.1 Anselme Payen (1838) was the first to recognize the composite nature of wood and referred to a carbon- rich substance as the “encrusting material” which embedded cellulose in the wood. Schulze (1865) later defined this encrusting material as lignin. Lignin has been described as a random, three-dimensional network polymer comprised of variously linked phenylpropane units.2 Lignin is the second most abundant biological material on the planet, exceeded only by cellulose and hemicellulose, and comprises 15-25% of the dry weight of woody plants. This macromolecule plays a vital role in providing mechanical support to bind plant fibers together. Lignin also decreases the permeation of water through the cell walls of the xylem, thereby playing an intricate role in the transport of water and nutrients. Finally, lignin plays an important function in a plant’s natural defense against degradation by impeding penetration of destructive enzymes through the cell wall. Although lignin is necessary to trees, it is undesirable in most chemical papermaking fibers and is removed by pulping and bleaching processes. 1.1.1 Biosynthesis Plant lignins can be broadly divided into three classes: softwood (gymnosperm), hardwood (angiosperm) and grass or annual plant (graminaceous) lignin.3 Three different phenylpropane units, or monolignols, are responsible for lignin biosynthesis.4 Guaiacyl lignin is composed principally of coniferyl alcohol units, while guaiacyl-syringyl lignin contains monomeric units from coniferyl and sinapyl alcohol. -

Black Liquor Gasification
Black Liquor Gasification Summary and Conclusions from the IEA Bioenergy ExCo54 Workshop This publication provides Wood and Wastes the record of a workshop organised by IEA Bioenergy. CO2 Pool Black liquor gasification is an interesting option for production of synthesis gas that can subsequently be converted to a variety of motor fuels. The technology can be integrated into modern, Carbon ecocyclic, kraft pulp mill Dioxide Pulp and Paper biorefineries. USA and Sweden lead developments in this field. It is of interest primarily among countries with strong pulp and paper industries and national policies which promote substitution of petrol and diesel by biofuels. IEA Bioenergy IEA BIOENERGY: ExCo:2007:03 INTRODUCTION a large pulp and paper industry. It is thus of great interest to convert the primary energy in the black liquor to an This publication provides a summary and the conclusions energy carrier of high value. from a workshop organised by IEA Bioenergy. It was held in conjunction with the 54th meeting of the Executive Worldwide, the pulp and paper industry currently processes Committee in Ottawa on 6 October 2004. The purpose of the about 170 million tonnes of black liquor (measured as dry workshop was to present the developments of black liquor solids) per year, with a total energy content of about 2EJ, gasification for the production of energy and/or biofuels making black liquor a very significant biomass source (see for transport and discuss the remaining barriers, either Figure 1). In comparison with other potential biomass technical or strategic, that need to be overcome in order sources for chemicals production, black liquor has the to accelerate the successful demonstration of black liquor great advantage that it is already partially processed and gasification technologies and subsequently their penetration exists in a pumpable, liquid form. -

Lime Green Spirit Activities
May is Mental Health Matters Month Lime Green Spirit Activities Mental Health Matters Month is a great opportunity to involve the people in your community to learn about mental health. Below is a creative way to incorporate lime green and promote mental health awareness. For a list of other activities, click here. Limeade and Ribbon Cookies: Create an area to pass out limeade beverages and lime green ribbon cookies. This unique way to engage with the community will provide an opportunity to pass out mental health information and discuss the importance of mental health. Other ideas include a mental health bake sale or bringing in treats to an office party or staff meeting. Ask to speak for a few minutes about what the lime green ribbon means to you and hang a poster on the wall to highlight Mental Health Matters Month. Ingredients Sugar Cookies Limeade Recipe 1 ½ cups butter, 1. In a large bowl, cream together butter and sugar For a tall pitcher of limeade, simply stir together: softened until smooth. Beat in eggs and vanilla. Stir in 1 cup fresh squeezed lime juice flour, baking powder and salt. Cover and chill 2 cupsIngredients white sugar Instructions (from about 8 to 10 limes) 1.5 cups butter, softened dough1. In a for large at bowl, least cream one together hour. butter and sugar 2 cups white sugar until smooth. Beat in eggs and vanilla. Stir in flour, 4 eggs4 eggs 4 cups water 1 tsp vanilla extract 2. Preheatbaking powderoven to and 400˚. salt. CoverRoll outand chilldough dough on for a floured 1 tsp vanilla5 cups all-purpose extract flour at least one hour. -

Georgia Peach Martini
710 HUFFMAN MILL ROAD BURLINGTON NORTH CAROLINA 27215 336.584.0479 GRILL584.COM WINE, BEER & COCKTAILS Our wines have been meticulously chosen from the world’s most treasured wineries. This wine list combines familiar names along with new hidden treasures chosen exclusively for . Our balanced wine selection is designed to fully complement your dining experience. Upon your request, our knowledgeable staff will pair an appropriate wine with your meal in an effort to create a memorable and elegant dining experience. Feeaturedatured WWhiteshites Steeple Jack Chardonnay, Austrailia 5.95 24.00 The colour of pale straw with a green hue; aromas of peach, ripe melon and honeysuckle on the nose. A full, soft, stonefruit palate of peaches and nectarines intermingled with rockmelon, with a creamy texture and a lingering lime citrus fi nish. Lafage Cote Est, France 6.75 26.00 The Cotes Catalanes Cote d’Est (50% Grenache Blanc, 30% Chardonnay, 20% Marsanne, aged in stainless steel on lees) is beautifully crisp and pure, with juicy acidity giving lift to notions of buttered citrus, green herbs and honeyed minerality. Medium-bodied, lively and certainly delicious, it’s a fantastic meal starter. Feeaturedatured RRedseds Gravel Bar Alluvial Red Blend, Columbia Valley 8.95 36.00 This is a full-bodied red blend (30% Cabernet Sauvignon, 30% Merlot, 15% Cabernet Franc, 10% Malbec, 10% Petit Verdot) with vibrant fl avors of dried cherries, plum, toffee, chocolate and vanilla. The structure is rich, with bold tannins extending the fi nish. Block & Tackle Cabernet Sauvignon, California 7.75 31.00 A deep ruby color with a rich nose of blackberries, raspberries and hints of pepper. -

American Ft Forest & Paper 2QE5
A 1 , i American Ft Forest & Paper 2QE5; Association , - May 6, 2015 Via Hand Delivery and by Email to iones.iim(a^epa.gov Administrator Gina McCarthy (1101A) Office of the Administrator Environmental Protection Agency 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 Re: Reguest for Full Exemption of Four Pulping Chemicals from the TSCA Chemical Data Reporting Rule Reguirements Dear Administrator McCarthy: The American Forest & Paper Association (AF&PA) hereby petitions EPA to amend the Chemical Data Reporting rule (CDR), 40 C.F.R. Part 711, to exempt from all CDR requirements four pulping chemicals involved in the manufacture of paper and other pulp-based products. The four pulping chemicals are complex mixtures used in the kraft pulping process: • Sulfite Liquors and Cooking Liquors, white (CAS No. 68131-33-9) (white liquor) • Sulfite Liquors and Cooking Liquors, spent (CAS No. 66071-92-9) (black liquor) • Sulfite Liquors and Cooking Liquors, spent, oxidized (CAS No. 68514-09-0) (black liquor, oxidized) • Sulfite Liquors and Cooking Liquors, green (CAS No. 68131-30-6) (green liquor) Each of these substances is manufactured and recycled onsite in a continuous closed loop. EPA has an enormous amount of information about these pulping chemicals and little current interest in them, as their potential risks are well understood and adequately managed. 1101 K Street, N.W., Suite 700 • Was hington, D.C.20005 • (202) 463•2700 •afandpa.org May 6, 2015 Page 2 This petition first identifies the four pulping chemicals in greater detail and explains why the kraft chemical regeneration process has led to inflated production volumes for these pulping chemicals in CDR data. -

A Dynamic Na/S Balance of a Kraft Pulp Mill
A dynamic Na/S balance of a kraft pulp mill Modeling and simulation of a kraft pulp mill using WinGEMS En dynamisk Na/S balans av ett sulfatbruk Modellering och simulering av ett sulfatbruk i WinGEMS Per Andersson Faculty of Health, Science and Technology Department of Engineering and Chemical Science, Chemical Engineering, Karlstad University Master theisis, 30 credits Supervisors: Niklas Kvarnström (KAU), Maria Björk (Stora Enso), Rickard Wadsborn (Stora Enso) Examinat or: Lars Järnström (KAU) 2014 -01-08 Version : 2.0 Abstract The main scope of this thesis was to create a simulation model of a kraft pulp mill and produce a dynamic Na/S balance. The model was made in WinGEMS 5.3 and the method consisted of implementing a static Na/S balance from the mill and created a model that described this chemical balance. Input data from the mill was collected and implemented in the model. A number of different cases were simulated to predict the effects of different process changes over time, dynamic balances. The result from the static balance showed that the model can describes the mill case. The result from the dynamic simulation showed that the model can be used to predict the effect of process changes over shorter periods of time. Executive Summary In the kraft mill the chemical balance is of interest to minimize the production cost. Normally there is an excess of sulfur and low levels of sodium, compared to what the process requires. In the future, the pulp mill will most likely produce other products than just pulp. These new production processes will also most likely affect the sodium and sulfur balance and there is a need to be able to predict this change. -

M^Ittt of $I)Tlos!Opfip in CHEMISTRY
PHYSICO-CHEMICAL STUDIES ON HEAVY METALS IN INDUSTRIAL WASTES AND RIVER WATER DISSERTATION FOR M^ittt of $I)tlos!opfip IN CHEMISTRY BY RUBINA CHAUDHARY DEPARTMENT OF CHEMISTRY ALIGARH MUSLIM UNIVERSITY ALIGARH (INDIA) 1989 DS1531 /^: F-HJ-VES I CO'—CHEIM I C(=%L- OT'LJFJ T. ESS ON HEZi^SW METi^^LS I hJ I hJDLJSTR:: I s^^il W#=IISTIE:S (=mMO RIVER: WftTEFi A DISSERTATION SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMINTS FOR THE DEGREE OF MASTER OF PHILOSOPHY IN CHEMISTRY RUBINA CHAUDHARY Department of Chemistry Aligarh Muslim University ALIGARH 1989 rnone : unice : t)U4Z Z. H. College of Engineering & Technology DEPARTMENT OF APPLIED CHEMISTRY Aligarh Muslim University ALIGARH—202002 (INDIA) Ref. No. Dated CERTIFICATE This is to certify that the work des cribed in this dissertation is the original work of Miss Rubina Chaudhary, carried out under my supervision and guidance. She has fulfilled all the requirements for the award of M.Phil, degree under the Academic Ordinaces of the Aligarh Muslim University, Aligerh. The work included in this disser tation has not been submitted elsewhere. 6.11.1989 PROF. MOHAMI^AD AsJl-'-y' ^ Supervisor ACKNOWLEDGEME Hr I am extremely grateful to my Supervisor Professor Mohansnad Ajmal for his guidance/ advice and personal interest during the course of this work, without whose sympathetic and inspiring attitude, timely assistance and affectionate encouragement this work would not have emerged in its present form. My thanks are also due to Prof. K.T.Nasim, Chairman, Department of Applied Chemistry, Z.H. College of Engineering & Technology, Allgarh Muslim university, Aligarh, for providing necessary facilities. -

Paper Recycling Technology Detailed Part 1A
Paper Recycling Technology and Science Dr. Richard A. Venditti Paper Science and Engineering Forest Biomaterials Department North Carolina State University Lecture: Paper recycling and technology course introduction and objectives Dr. Richard Venditti Faculty member in the Paper Science and Engineering Program in the Forest Biomaterials Department at North Carolina State University PhD in Chemical Engineering, BS in Pulp and Paper Science and Chemical Engineering Research areas: � Paper recycling � Utilization of forest/agricultural materials for new applications � Life cycle analysis Named a TAPPI Fellow in 2012 Relevant research projects: – The detection of adhesive contaminants – The changes in fibers upon recycling – Automatic sorting of recovered papers – Flotation deinking surfactants – Agglomeration deinking – Screening phenomena and pressure sensitive adhesives – Deposition of adhesive contaminants – Neural networks to control deinking operations – Sludge conversion to bio-ethanol and to bio- materials Course Outline The US Paper Recycling Industry Recovered Paper Grades and Contaminants Effect of Recycling on Fibers/Paper Unit Operations � Pulping, Cleaning, Screening, Washing, Flotation, Dispersion, Bleaching, ….. Image Analysis, Deinking Chemicals System Design Advanced/Additional Topics Course Activities Viewing of the Videos of Lectures � Base lectures by Venditti � Guest lectures from industry leaders Homework assignments Final Exam Critical Issues in Recycling: Going deeper into the recovered paper stream -

Green Flowers
A Horticulture Information article from the Wisconsin Master Gardener website, posted 15 March 2010 Green Flowers Green is the color right now with St. Patrick’s Day approaching. Have you ever paid attention to fl owers with this color? In the garden, green is the most common foliage color, yet one of the rarest fl ower colors. Green fl owers are unusual, so when presented correctly, they add an air of sophistication to a planting and look good with every color. There are some naturally occurring green fl owers, but many more have been “created” by breeding and selection. These fl oral oddities are a fun addition to the garden. Some plants that naturally come in green include orchids and aroids Green is the thing in March. like Jack-in-the-Pulpit (Arisaema triphyllum) (not to mention a number of weeds with inconspicuous fl owers, but who wants those in their garden?). Naturally green fl owers of Cymbidium orchid (L), Pterostylis orchid (LC), Arisaema triphyllum (RC) and Bells of Ireland (R). Many newer green fl owers have been developed primarily for the cut fl ower and fl orist industry. There are green roses such as light green ‘Jade’ and yellow green ‘Emerald’ (and more varieties currently being developed in The Netherlands) as well as a bi-color rose ‘Cezanne’ that is a creamy pistachio edged with pink. The mossy green ‘Marimo’ hybrid Gerbera, green hydrangeas, green cymbidiums, and ‘Lime Green’ Lisianthus (from the Mariachi series that is a beautiful, pale green ) are marketed to fl orists. Among the exotic blooms shipped for fl oral arrangements are ‘Midori’ anthuriums and ‘Green Goddess’ callas (Zantedeschia).