A[Mof6](A =Li–Cs) Riane E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Certain Data Contained in This Document May Be Difficult to Read in Microfiche Products
NOTICE CERTAIN DATA CONTAINED IN THIS DOCUMENT MAY BE DIFFICULT TO READ IN MICROFICHE PRODUCTS. dSa./ NREL/MP-451-4778B . UC Category': 270 • DE92 NRHL/MP--451-4778B DE92 04032.1 Safety Analysi%^.eport For the Use of Hal|ardous Production Materials in Photovoltaic Applications at the National Renewable Energy Laboratory Volume II: Appendices •.-ft '4^ oil, R.S, Crandall B»P. Nelson P.D. Moskowitz V.M. Fthenakis *N?S! National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 A Division of Midwest Research Institute Operated for the U.S. Department of Energv under Contract No. DE-AC02-83CH10093 " July 1992 Hi ^_ oismiuuiiuh or IMUI NOTICE Tnis report was ptepared as an account of work sponsoied by an agency of the United States government Neither the United Slates government nor any agency thereof nor any of their employees, makes any warranty express o< implied or assumes any legal liability Of tesponsibility'or the accuracy completeness or usefulness of any information apparatus product or process disclosed 01 represents that its use would not infringe privately owned, rights Reference herein to any specie commercial product process or service by trade name trademark manufacturer or otherwise ooes not necessarily constitute or imply its endorsement recommendation or favoring by the United Slates government or any agency lhereo> The views and opinions of authors expresseo herein do not necessarily state or reflect those of the United State; government or any agency theieof APPENDICES - TABLE OF CONTENTS A: AffCJtiftflt/RisK -

Method for Preparing Gaseous Metallic Fluoride
Europaisches Patentamt 0 333 084 J European Patent Office (p) Publication number: A2 Office europeen des brevets EUROPEAN PATENT APPLICATION @ Application number: 89104364.8 @) lnt.Cl.4:C01B 9/08 © Date of filing: 11.03.89 Priority: 16.03.88 JP 60271/88 Applicant: MITSUI TOATSU CHEMICALS INC. 16.03.88 JP 60272/88 No. 2-5, Kasumigaseki 3-chome 16.03.88 JP 60273/88 Chiyoda-Ku Tokyo 100(JP) 16.03.88 JP 60274/88 11.11.88 JP 283749/88 Inventor: Harada, Isao, Mr. 5-7-2, Hikoshima Sakomachi Date of publication of application: Shimonoseki-shi Yamaguchi-ken(JP) 20.09.89 Bulletin 89/38 Inventor: Yoda, Yukihiro, Mr. 6-1-23, Hikoshima Sakomachi Designated Contracting States: Shimonoseki-shi Yamaguchi-ken(JP) DE FR GB IT Inventor: iwanaga, Naruyuki, Mr. 5-7-1, Hikoshima Sakomachi Shimonoseki-shi Yamaguchi-ken(JP) Inventor: Nishitsuji, Toshihiko, Mr. 5-9-6-505, Hikoshima Sakomachi Shimonoseki-shi Yamaguchi-ken(JP) Inventor: Kikkawa, Akio, Mr. M-408, 3-1, Shinakada Nishimachi Shimonoseki-shi Yamaguchi-ken(JP) Representative: Schiiler, Horst, Dr. Kaiserstrasse 69 D-6000 Frankfurt/Main 1(DE) © Method for preparing gaseous metallic fluoride. © The method for preparing a gaseous metallic fluoride is here disclosed which comprises reacting a metal or its oxide with a fluorine gas or nitrogen trifluoride gas, the aforesaid method being characterized by comprising the steps of mixing the metal or its oxide with a molding auxiliary comprising a solid metallic fluoride which does I not react with fluorine and nitrogen trifluoride; molding the resulting mixture under pressure; and contacting the [molded pieces with the fluorine gas or nitrogen trifluoride gas, while the molded pieces are heated. -

The Study of Alternate, Solid-Phase Fluorinating Agents for Use in Reactive Gas Recycle of Used Nuclear Fuel
University of South Carolina Scholar Commons Theses and Dissertations 2013 The tudS y of Alternate, Solid-Phase Fluorinating Agents for Use in Reactive Gas Recycle of Used Nuclear Fuel Dillon Inabinett University of South Carolina Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the Nuclear Engineering Commons Recommended Citation Inabinett, D.(2013). The Study of Alternate, Solid-Phase Fluorinating Agents for Use in Reactive Gas Recycle of Used Nuclear Fuel. (Master's thesis). Retrieved from https://scholarcommons.sc.edu/etd/3587 This Open Access Thesis is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. THE STUDY OF ALTERNATE, SOLID-PHASE FLUORINATING AGENTS FOR USE IN REACTIVE GAS RECYCLE OF USED NUCLEAR FUEL by Dillon Inabinett Bachelor of Science University of South Carolina, 2012 Submitted in Partial Fulfillment of the Requirements For the Degree of Master of Science in Nuclear Engineering College of Engineering and Computing University of South Carolina 2013 Accepted by: Travis W. Knight, Major Professor Joshua Gray, Second Reader Lacy Ford, Vice Provost and Dean of Graduate Studies © Copyright by Dillon Inabinett, 2013 All Rights Reserved. ii DEDICATION I would like to dedicate this work to my mother, for her endless support throughout all of my endeavors. Though life may not have always been perfect, the one thing that remains true is her love and affection. iii ACKNOWLEDGEMENTS I would like to thank my two advisors, Dr. Travis Knight and Dr. -

Hazardous Waste Management Guidebook
Hazardous Waste Management Guidebook FOR UNIVERSTIY AT BUFFALO CAMPUS LABORATORIES Prepared By Environment, Health & Safety Services 220 Winspear Avenue Buffalo, NY 14215 Phone: 716-829-3301 Web: www.ehs.buffalo.edu UB EH&S Hazardous Waste Management Guidebook Table of Contents 1.0 PURPOSE ........................................................................................... 3 2.0 SCOPE ............................................................................................... 4 3.0 DEFINITIONS ...................................................................................... 4 4.0 RESPONSIBILITIES ............................................................................... 5 4.1 EH&S ................................................................................................... 5 4.2 Faculty, Staff, and Students ............................................................. 5 5.0 PROCEDURES ....................................................................................... 6 5.1 Hazardous Waste Determination ................................................... 6 5.1.1 Characteristic Hazardous Wastes .......................................... 7 5.1.2 Listed Hazardous Wastes ........................................................ 9 5.2 Satellite Accumulation of Hazardous Waste ............................ 9 5.2.1 Accumulation Areas ............................................................. 10 5.2.2 Requirements for Hazardous Waste Containers ................ 10 5.2.3 Segregation of Hazardous Wastes ..................................... -

Ornl-Tm-1976
OAK RIDGE NATIONAL LABORATORY operated by I UNION CARBIDE CORPORATION a-: 1 NUCLEAR DIVISION a!a for the U.S. ATOMIC ENERGY COMMISSION ORNL- TM- 1976 A LITERATURE SURVEY OF THE FLUORIDES AND OXYFLUORIDES OF MOLYBDENUM C. F. Weaver H. A. Friedman NOTICEThis d ocument contains information of a preliminary nature and war prepared primarily for internal use at the Oak Ridge National Laborotory. It is subject to revision or correction and therefore does not represent a final report. rLEGAL NoT’CE/ This report WQI prepared os an account of Government sponsored work. Neither the United States, nor the Commtssion, nor any powon acting on behalf of the Commission: A. Makes ony worronty or representation, expressed or implied, with respect to the accuracy, completeness, or usefulness of the information contained in this report, or that th* “se of ony information, opparatut, method, or process disclosed in this report moy not infringe privately owned rights; or l3. Assumes ony liabilities with respect to the use of, or for damages resulting from tlm use of any information, apparatus, method, M process disclosed in this repopt. As usad in the above, “person acting on behalf of the Commisriou” includes any employee or contractor of the Conmission, or employee of such contractor, tc th+ extent thot sxh employee oi contractor of the Commission, or amploycs of such controctcr prepares, disseminates, or provides access to, ony information pursuant to his smploymont or contract with the Commission, or his employment with such contractor. V . ORNL-TM-1976 Contract No. W-7405-eng-26 REACTOR CHEMISTRY DIVISION A LITERATURE SURVEY OF THE FLUORIDES AND OXYFLUORIDES OF MOLYBDENUM C. -

Modelling of the Deposition of Molybdenum on Silicon from Molybdenum Hexafluoride and Hydrogen E
Modelling of the Deposition of Molybdenum on Silicon from Molybdenum Hexafluoride and Hydrogen E. Orij, M. de Croon, G. Marin To cite this version: E. Orij, M. de Croon, G. Marin. Modelling of the Deposition of Molybdenum on Silicon from Molyb- denum Hexafluoride and Hydrogen. Journal de Physique IV Proceedings, EDP Sciences, 1995,05 (C5), pp.C5-331-C5-338. 10.1051/jphyscol:1995539. jpa-00253866 HAL Id: jpa-00253866 https://hal.archives-ouvertes.fr/jpa-00253866 Submitted on 1 Jan 1995 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C5, supplkment au Journal de Physique 11, Volume 5, juin 1995 Modelling of the Deposition of Molybdenum on Silicon from Molybdenum Hexafluoride and Hydrogen E.N. Orij, M.H.J.M. de Croon and G.B. Marin Eindhoven Universiry of Technology, Laboratorium voor Chemische Technology, P.O. Box 513, 5600 MB Eindhoven, The Netherlands Abstract: The deposition of molybdenum on silicon from MoF, and H, is studied using a microbalan- ce setup. The deposition rate is time dependent, which is explained by the significant contribution of the reduction of MoF, by Si. -

Tables of Molecular Vibrational Frequencies, Consolidated Volume I
NAT L INST. OF STAND & TECH R.I.C. NBS PUBLICATIONS DATE DUE f i tZ x tx AMP \ Q 1998 Demco, Inc. 38-293 OF STANDARDS LIBRARY UNITED STATES DEPARTMENT OF COMMERCE JUL 2 7 1976 762636 Peter G. Peterson, Secretary C, NATIONAL BUREAU OF STANDARDS • Lawrence M. Kushner, Acting Director ( ; :* no ‘ 3 / 9 7^ Tables of Molecular Vibrational Frequencies Consolidated Volume I Takehiko Shimanouchi Department of Chemistry Faculty of Science University of Tokyo Tokyo, Japan NSRDS-NBS 39 Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.), 39, 164 pages (June 1972) L NSRDAP © 1972 by the Secretary of Commerce on Behalf of the United States Government Supersedes and extends the data contained in Tables of Molecular Vibrational Frequencies, NSRDS-NBS-6, Part 1; NSRDS-NBS-11, Part 2; and NSRDS-NBS-17, Part 3. Issued June 1972 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 (Order by SD Catalog No. C13. 48:39). Price S3 Stock Number 0303—0845 Library of Congress Catalog Card Number: 66-60085 II Foreword The National Standard Reference Data System provides effective access to the quantitative data of physical science, critically evaluated and compiled for convenience, and readily accessible through a variety of distribution channels. The System was established in 1963 by action of the President’s Office of Science and Technology and the Federal Council for Science and Technology, with responsibility to administer it assigned to the National Bureau of Standards. The System now comprises a complex of data centers and other activities, carried on in academic institutions and other laboratories both in and out of government. -
Download (7MB)
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] RADIOTRACER STUDIES OF THE FLUORINE BOND LABILITY OF SOME BINARY FLUORIDES. Thesis submitted to the University of Glasgow in fulfilment of the requirement of the degree of Doctor of Philosophy. by MOHAMMED FOUZI GHORAB M.Sc. Department of Chemistry University of Glasgow September, 19S8. ProQuest Number: 10998195 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10998195 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLC. -

State-Of-The-Art Report on the Progress of Nuclear Fuel Cycle Chemistry
Nuclear Science 2018 State-of-the-Art Report on the Progress of Nuclear Fuel Cycle Chemistry Nuclear Science State-of-the-Art Report on the Progress of Nuclear Fuel Cycle Chemistry © OECD 2018 NEA No. 7267 NUCLEAR ENERGY AGENCY ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT The OECD is a unique forum where the governments of 35 democracies work together to address the economic, social and environmental challenges of globalisation. The OECD is also at the forefront of efforts to understand and to help governments respond to new developments and concerns, such as corporate governance, the information economy and the challenges of an ageing population. The Organisation provides a setting where governments can compare policy experiences, seek answers to common problems, identify good practice and work to co-ordinate domestic and international policies. The OECD member countries are: Australia, Austria, Belgium, Canada, Chile, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Latvia, Luxembourg, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, the United Kingdom and the United States. The European Commission takes part in the work of the OECD. OECD Publishing disseminates widely the results of the Organisation’s statistics gathering and research on economic, social and environmental issues, as well as the conventions, guidelines and standards agreed by its members. This work is published on the responsibility of the Secretary-General of the OECD. NUCLEAR ENERGY AGENCY The OECD Nuclear Energy Agency (NEA) was established on 1 February 1958. -

Pp-03-25-New Dots.Qxd 10/23/02 2:38 PM Page 558
pp-03-25-new dots.qxd 10/23/02 2:38 PM Page 558 558 MENDELEVIUM MENDELEVIUM [7440-11-1] Symbol: Md; atomic number 101; atomic weight (most stable isotope) 257; a man-made radioactive transuranium element; an inner-transition element of actinide series; electron configuration [Rn]5f137s2; valence +2, +3. Isotopes, half-lives and their decay modes are: Mass Half-life Decay Mode Md–252 8 min Orbital electron capture Md–255 30 min Orbital electron capture Md–256 1.5 hr. Orbital electron capture Alpha decay Md–257 3.0 hr. Orbital electron capture Alpha decay Md–258 60 day Alpha decay History The element first was made by Ghiorso, Harvey, Choppin, Thompson, and Seaborg in 1955 in Berkeley, California. It was synthesized by bombardment of einsteinium-253 with alpha particles of 41 MeV energy in a 60-inch cyclotron. The element was named Mendelevium in honor of Russian chemist Dimitri Mendeleev. Mendelevium –258 isotope with a half-life of 60 days was discovered in 1967. The element has no commercial use except in research to synthesize isotopes of other transuranium elements. Synthesis Mendelevium was synthesized first by bombarding Einstein-253 with heli- um ions. The nuclear reaction is: 253 +4 →256 + 1 99 Es 2 He 101Md 0 n All isotopes of medelevium have been synthesized by other nuclear reac- tions since its discovery. They are prepared by bombarding uranium, ein- steinium, and californium isotopes with heavy ions, such as boron-11, carbon- 12 and carbon-13. 238 +19 →252 + 1 92UF9 101 Md 50 n For example, uranium-238 when bombarded with fluorine-19 produced Md- 252. -

United States Patent 19 (11) 3,806,579 Carles Et Al
United States Patent 19 (11) 3,806,579 Carles et al. (45) Apr. 23, 1974 (54) METHOD OF PURIFICATION OF URANUM 3,425,812 2/1969 Cousin et al.......................... 23/352 HEXAFLUORIDE 3,359,078 12/1967 Alter et al............................. 23/326 3,340,019 9/1967 Pierini et al.......................... 23/326 (75) Inventors: Maurice Carles; Jacky Fra, both of Pierrelatte, France Primary Examiner-Carl D. Quarforth 73 Assignee: Commissariat A L'Energie Assistant Examiner-F. M. Gittes Atomique, Paris, France Attorney, Agent, or Firm-Cameron, Kerkam & Sutton 22 Filed: Mar. 22, 1971 21 Appl. No.: 126,682 57 ABSTRACT This invention relates to a method of purification of 30 Foreign Application Priority Data uranium hexafluoride contaminated with impurities such as molybdenum hexafluoride and tungsten hexa Apr. 8, 1970 France.............................. 70. 12685 fluoride. (52) U.S. Cl......................... 423/19, 42318, 423/258 The contaminated uranium hexafluoride is distilled 51 Int. Cl.......................... a a P A 8 is a C01g 43/06 from the liquid phase in the presence of chlorine (58) Field of Search........ 23/326,339, 352; 423/19, trifluoride which forms an azeotrope with the 423/258, 8 impurities to be removed from the uranium hexafluoride, said impurities are withdrawn from the 56) References Cited top of the column and the purified uranium UNITED STATES PATENTS hexafluoride is collected at the bottom of the column. 2,830,873 4/1958 Katz et al.............................. 23/326 1 Claim, 4 Drawing Figures 'ATENTED APR 23 1974 3,806,579 1. 2 METHOD OF PURIFICATION OF URANIUM nium diuranate by bubbling of uranium hexafluoride HEXAFLUORDE through ammonia, dissolving the ammonium diuranate which is formed with nitric acid, extraction of the ura This invention relates to a method of purification of nyl nitrate by tributylphosphate whilst the impurities uranium in the form of uranium hexafluoride which 5 remain in the aqueous phase, stripping of the uranium contains impurities such as molybdenum and tungsten. -
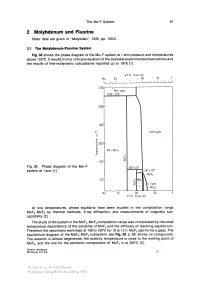
2 Molybdenum and Fluorine Older Data Are Given in "Molybdän", 1935, Pp
The Mo-F System 67 2 Molybdenum and Fluorine Older data are given in "Molybdän", 1935, pp. 150/3. 2.1 The Molybdenum-Fluorine System Fig. 38 shows the phase diagram of the Mo-F system at 1 atm pressure and temperatures above 150°C. It results from a eritieal evaluation of the available experimental observations and the results of thermodynamie ealeulations reported up to 1978 [1]. wt % tluorlne Mo 30 50 70 F ~,LI __-L __ L--L~I~~-LI ~I_I~I~I~I~II lZ00 ~ Mo+gas 1100! ZOO 1000 c- 800 I- 1 atm gas c -2::: 600 I- w 0.. E Mo +MoFl w ~ m u- D ::E 400 I- Fig.38. Phase diagram of the Mo-F 300! 40' system at 1 atm [1]. ........ Z67! 10' 200 c- u:' MoF4 D + ::E ~:F'L+gas L Mo 16 ~ I Mo 70 80 90 ot % tluorlne At low temperatures, phase equilibria have been studied in the eomposition range MoFr MoF6 by thermal methods, X-ray diffraetion, and measurements of magnetie sus eeptibility [2]. The study of the system in the MoFrMoFs eomposition range was eomplieated by the small temperature dependenee of the solubility of MoF4 and the diffieulty of reaehing equilibrium. Therefore the speeimens were kept at 100 to 150°C for 10 to 12 h. MoFs also forms a glass. The equilibrium diagram of the MoFrMoFs subsystem, see Fig. 39, p. 68, shows no eompounds. The euteetie is alm ost degenerate, the euteetie temperature is elose to the melting point of MoFs, and the line for the periteetie eomposition of MoF4 is at 300°C [2].