Background Notes on the Physics of Steam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho a Dissertation
Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems By Tony Ho A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering – Mechanical Engineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair Professor Van P. Carey Professor Per F. Peterson Spring 2012 Abstract Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho Doctor of Philosophy in Mechanical Engineering University of California, Berkeley Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially increase power generation and improve the utilization efficiency of renewable energy and waste heat recovery systems. A brief review of current advanced vapor power cycles including the Organic Rankine Cycle (ORC), the zeotropic Rankine cycle, the Kalina cycle, the transcritical cycle, and the trilateral flash cycle is presented. The premise and motivation for the OFC concept is that essentially by improving temperature matching to the energy reservoir stream during heat addition to the power cycle, less irreversibilities are generated and more power can be produced from a given finite thermal energy reservoir. In this study, modern equations of state explicit in Helmholtz energy such as the BACKONE equations, multi-parameter Span- Wagner equations, and the equations compiled in NIST REFPROP 8.0 were used to accurately determine thermodynamic property data for the working fluids considered. Though these equations of state tend to be significantly more complex than cubic equations both in form and computational schemes, modern Helmholtz equations provide much higher accuracy in the high pressure regions, liquid regions, and two-phase regions and also can be extended to accurately describe complex polar fluids. -

International Steam Tables - Properties of Water and Steam Based on the Industrial Formulation IAPWS-IF97
International Steam Tables - Properties of Water and Steam based on the Industrial Formulation IAPWS-IF97 Tables, Algorithms, Diagrams, and CD-ROM Electronic Steam Tables - All of the equations of IAPWS-IF97 including a complete set of supplementary backward equations for fast calculations of heat cycles, boilers, and steam turbines Bearbeitet von Wolfgang Wagner, Hans-Joachim Kretzschmar Neuausgabe 2007. Buch. xix, 391 S. ISBN 978 3 540 21419 9 Format (B x L): 21 x 27,9 cm Weitere Fachgebiete > Geologie, Geographie, Klima, Umwelt > Umweltwissenschaften > Ökologie, Allgemeines Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Preface to the Second Edition The international research regarding the thermophysical properties of water and steam has been coordinated by the International Association for the Properties of Water and Steam (IAPWS). IAPWS is responsible for the international standards for thermophysical properties. These standards and recommendations are given in the form of releases, guidelines, and advisory notes. One of the most important standards in this sense is the formulation for the thermodynamic properties of water and steam for industrial use. In 1997, IAPWS adopted the “IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam” for industrial use, called IAPWS-IF97 for short. The formulation IAPWS-IF97 replaced the previous industrial formulation IFC-67 published in 1967. -

Water: Economics and Policy 2021 – ECI Teaching Module
Water: Economics and Policy By Brian Roach, Anne-Marie Codur and Jonathan M. Harris An ECI Teaching Module on Social and Environmental Issues in Economics Global Development Policy Center Boston University 53 Bay State Road Boston, MA 02155 bu.edu/gdp WATER: ECONOMICS AND POLICY Economics in Context Initiative, Global Development Policy Center, Boston University, 2021. Permission is hereby granted for instructors to copy this module for instructional purposes. Suggested citation: Roach, Brian, Anne-Marie Codur, and Jonathan M. Harris. 2021. “Water: Economics and Policy.” An ECI Teaching Module on Social and Economic Issues, Economics in Context Initiative, Global Development Policy Center, Boston University. Students may also download the module directly from: http://www.bu.edu/eci/education-materials/teaching-modules/ Comments and feedback from course use are welcomed: Economics in Context Initiative Global Development Policy Center Boston University 53 Bay State Road Boston, MA 02215 http://www.bu.edu/eci/ Email: [email protected] NOTE – terms denoted in bold face are defined in the KEY TERMS AND CONCEPTS section at the end of the module. 1 WATER: ECONOMICS AND POLICY TABLE OF CONTENTS 1. GLOBAL SUPPLY AND DEMAND FOR WATER.................................................... 3 1.1 Water Demand, Virtual Water, and Water Footprint ................................................. 8 1.2 Virtual Water Trade ................................................................................................. 11 1.3 Water Footprint the Future of Water: -

Water Withdrawal and Conservation Practices
Water Withdrawal And Conservation Practices Prepared for Michigan Chamber of Commerce February 2008 Submitted by Barr Engineering Company Table of Contents 1. Introduction 1 Foreword ..................................................................................................................................... 1 Background and Purpose ............................................................................................................. 1 2. Position Statement 3 Water Efficiency – Resource Sustainability – Conservation Management................................... 3 Our Goal Statement ..................................................................................................................... 3 Our Objectives............................................................................................................................. 3 3. Generally Accepted Management Practices for Water Efficiency and Conservation 4 Communication ........................................................................................................................... 4 Process......................................................................................................................................... 4 Washrooms.................................................................................................................................. 5 Landscaping................................................................................................................................. 5 Appendix A – Links to Websites 6 Appendix -

Published by Ministry of Energy and Water Development February 2010
- NATIONAL WATER POLICY Published by Ministry of Energy and Water Development February 2010 TABLE OF CONTENTS FOREWORD ......................................................................................................................... i ACKNOWLEDGEMENT ....................................................................................................... ii ACRONYMS .........................................................................................................................iii WORKING DEFINITIONS ....................................................................................................iv 1. INTRODUCTION ............................................................................................................ 1 1.1 Water Resources Availability .............................................................................................. 1 1.2 Rainfall Situation................................................................................................................... 2 1.3 Surface Water Situation ...................................................................................................... 3 1.4 Groundwater Situation ......................................................................................................... 3 1.5 Environmental Management .............................................................................................. 4 1.6 Historical Background .......................................................................................................... 5 2. SITUATION -

Steam Water Heaters
Issue 82 Nov 2018 Steam Water Heaters Introduction provide corrosion resistance as well Water heaters are available which utilize various sources of energy, as suitability for potable water including electricity, steam, fuel gas, geothermal (heat pump) and applications. Accurate hot water solar. Today, the most commonly used energy source for domestic temperature control is provided water heating is electricity, followed by natural gas. At the NIH using modulating fail-safe campus in Bethesda, steam is the source of energy used to heat pneumatically actuated or electric modulating fast positioning (with domestic and laboratory water as well as numerous other applications. Alternate heating sources for water heaters may only position feedback type) control be used for special applications and with pre-approval by ORF. valves. NIH requires that such water temperature control shall heat the A Brief Evolution of Water Heating water to 60°C–63°C (140°F–145°F) The first water heater was invented in 1868, which lead to the and tempered down to 52°C–54°C invention of the first storage tank-type gas water heater in 1889. At (125°F–130°F) for general potable that time fossil fuels such as natural gas, oil, and coal were water system distribution by a commonly used to heat water. Then, as electricity became American Society of Safety Engineer commercially available electrically-powered water heaters grew to (ASSE) 1017 master thermostatic be popular, assisted by their ease of installation and low first cost. mixing valve (arranged in parallel to As it became more commonplace, heated water was utilized for provide N+1 redundancy). -
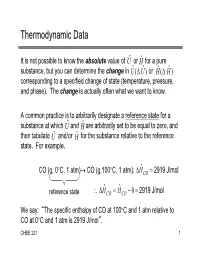
Thermodynamic Data
Thermodynamic Data It is not possible to know the absolute value of U ˆ or H ˆ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or Hˆ ( Hˆ ) corresponding to a specified change of state (temperature, pressure, and phase). The change is actually often what we want to know. A common practice is to arbitrarily designate a reference state for a substance at which U ˆ and H ˆ are arbitrarily set to be equal to zero, and then tabulate U ˆ and/or H ˆ for the substance relative to the reference state. For example, ˆ CO (g, 0C, 1 atm) CO (g,100C, 1 atm): HCO 2919 J/mol ˆ ˆ reference state HCO HCO 0 2919 J/mol We say: “The specific enthalpy of CO at 100C and 1 atm relative to CO at 0C and 1 atm is 2919 J/mol”. CHEE 221 1 Reference States and State Properties Most (all?) enthalpy tables report the reference states (T, P and State) on which the values of H ˆ are based; however, it is not necessary to know the reference state to calculate H (change in enthalpy) for the transition from one state to another. ˆ ˆ –H from state 1 to state 2 equals H 2 H 1 regardless of the reference state upon which ˆ and ˆ were based H1 H 2 – Caution: If you use different tables, you must make sure they have the same reference state This result is a consequence of the fact that H ˆ (and U ˆ ) are state properties, that is, their values depend only on the state of the species (temperature, pressure, state) and not on how the species reached its state. -

Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations
Backpressure Steam Power Generation in District Energy and CHP - Energy Efficiency Considerations A 1st Law, 2nd Law and Economic Analysis of the Practical Steam Engine in a District Energy/CHP Application Joshua A. Tolbert, Ph.D. Practical Steam Energy Efficiency Engineering – How is efficiency calculated? • What is the Status Quo? – Engineers currently focus on limiting energy losses as the primary point of focus for district energy and CHP systems – Only local energy losses are typically considered causing energy efficiency opportunities to be wasted • Why? – Conventional wisdom tells us that minimizing local energy losses is ultimate goal • How should we change? – Could a different approach improve global efficiency? Motivation Common Questions: 1. How can installing an imperfect device in parallel to an isenthalpic pressure reducing valve (PRV) improve efficiency? 2. My analysis shows an incremental fuel cost with PRV parallel, how can this be more efficient? Analysis Overview • Consider a Practical Steam Engine (PSE) in a Pressure Reducing Valve (PRV) parallel district energy application • PSE operation is consistent with CHP application • Consider 1st Law, 2nd Law and economic analysis for energy efficiency • Draw conclusions Backpressure Application – Case Study Enwave Seattle - District Energy / CHP Application Assumptions Equipment • 1.5 MW (5.118 MMBtu/hr) • Practical Steam Engine (PSE) heating load is considered o Isentropic efficiency of 80% • Condensate exiting load: o Mechanical efficiency of 80% Saturate liquid at 20 psig o Generator efficiency of 95% • No heat losses in piping or • Isenthalpic PRV equipment* • 150 psig saturated steam boiler • 80% boiler and feedwater pump efficiency *Incorporating actual heat losses does not significantly affect results of analysis. -

Superheated Steam in Locomotive Service
I LL INO I S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. 0 ~0' / ~ ( ~ 1~CC~& '~f~LW "0~ "N ~ ~- ~N~TV N Q SC 2"' C' ''~~C'~ C" 4 AA.A..~ Ay ~4.A A A'-' 'A ' ~ ' ,~A~A 1 A' 'A": "I ~' ~A 9 ~AA >' 0 -A.'-- ~ A" Z 'A A' * A -A "* K~ A. A' ~. 'A A, ,~, 'U",' 4 *, A' '~'" 'A * JA A' 'A' A A K 'A 'A / A A A' 'A vas,^esta>bflibea~ ~ by ^f'A A' A ,rt~7B A 'A on, investigations~ve~Wg~on.~. A' ~ 'i* *t~.,to studysti~dy~ prob1~m~problems A sS ~4 IOAWeto eadA manu-m&i~n- 'be industrial interests 'A' of theb8tat;' -'$a te' The control of tho Engineering ExperintSerment• 'titionSl i•is is'vested vested 'A UNIVERSITY OF ILLINOIS ENGINEERING EXPERIMENT STATION BULLETIN No. 57 APRIL 1912 SUPERHEATED STEAM IN LOCOMOTIVE SERVICE (A REVIEW OF PUBLICATION NO. 127 OF THE CARNEGIE INSTITUTION OF WASHINGTON) BY W. F. M. GOSS DEAN OF THE COLLEGE OF ENGINEERING DIRECTOR OF THE ENGINEERING EXPERIMENT STATION DIRECTOR OF THE SCHOOL OF RAILWAY ENGINEERING AND ADMINISTRATION CONTENTS PAGE I. Introduction-A Summary of Conclusions .......... 3 II. Foreign Practice in the Use of Superheated Steam in Locomotive Service.............. ......... 5 III. Tests to Determine the Value of Superheating in Lo- comotive Service................ .. .. .... 14 IV. Performance of Boiler and Superheater.. ........... 20 V. Performance of the Engine and of the Locomotive as a W hole... ............ .. .. ........ 35 VI. Economy Resulting from the Use of Superheated Steam ........... -

Evaluation of Hydrologic Alteration and Opportunities for Environmental Flow Management in New Mexico
Evaluation of Hydrologic Alteration and Opportunities for Environmental Flow Management in New Mexico October 2011 Photo: Elephant Butte Dam, Rio Grande, New Mexico; Prepared by The Cadmus Group, Inc. Courtesy U.S. Bureau of Reclamation U.S. EPA Contract Number EP-C-08-002 i Table of Contents Executive Summary ............................................................................................................................................................ 1 1. Introduction ................................................................................................................................................................ 3 2. Hydrologic Alteration Analysis Study Design ....................................................................................................... 5 What Sites Are Assessed? ............................................................................................................................. 5 What Drives Hydrologic Alteration? ........................................................................................................ 11 How Is Hydrologic Alteration Assessed?................................................................................................. 16 3. Results of Hydrologic Alteration Analysis ........................................................................................................... 19 Alteration of High Flow Events ................................................................................................................ 19 Alteration of Low Flow -

CONCORD STEAM CORPORATION Year Ended DECEMBER 31, 2016
NHPUC Page2 Annual Report of CONCORD STEAM CORPORATION Year Ended DECEMBER 31, 2016 PUBLIC UTILITIES COMMISSION CONCORD I ANNUAL REPORT OF Concord Steam Corporation FOR THE YEAR ENDED 2016 Offrcer to whom correspondence should be addressed regarding this report: Name Peter Bloomfield Title President Address PO Box 2520, Concord, NH 03302-2520 Sheetl NHPUC Page 2 Annual Report of CONCORD STEAM CORPORATION Year Ended DECEMBER 31, 2016 PUBLIC UTILITIES COMMISSION CONCORD 15 ffPR'1? åHË:84 ,irlrèi.i,.l ¡-,:-l'.r'.' ir:it i !) ¿{ Y-, 4t 4) /, ANNUAL REPORT OF ¡'- 4 C Steam C on ,',i,r/ri# A{rr¡ ,a I itn FO THE YEAR ENDED Nt/ 20t7 2015 Officer to whom conespondence should be addressed regarding this report: Name Peter Bloomfield Tirle President Address PO Box 2520, Concord, NH 03302-2520 Sheetl INDEX Table Page Table Page A N Abandoned Property 13 104 New Business and Sales Expenses 43 206 Accounts Receivable 2l 107 Non-operating Property t2 104 Affi I iates-investments in 15 105 Notes-long term debt 26 r09 -payables to ll0 -payable 27 il0 -receivables from 22 107 o B Oath 401 Balance Sheet l0 100 Officers, list of 42-S J Bonded Indebtedness 26 r09 Operating Expenses-summary 42 203 -detail 42 204 C Operating Revenues 41 203 Capital, Fixed, Accounts n 102 CapitalStock 25 r09 P Capital Surplus 34 I l3 Payables-notes a1 ll0 Credits, Miscel laneous, Unadjusted 32 n2 -to affiliates 28 ll0 Payments to Individuals 5 D Prepayments t8 106 Debt-Long Term 26 109 Property-abandoned t3 104 -unamortized discount and expens 23 108 -for future development -

View the Manual
Manual Epilepsy Warning Some people may experience photosensitive epileptic seizures or a loss of consciousness when viewing certain visual stimuli, such as flashing lights or patterns, in everyday life. Such individuals are at risk of experiencing seizures while watching television or playing video games. This can happen even to individuals with no history of related health conditions or signs of epilepsy. The following symptoms are characteristic of photosensitive seizures: blurred vision, eye or facial twitching, trembling arms or legs, disorientation, confusion or momentary loss of balance. During a photosensitive seizure, loss of consciousness and convulsions may cause serious accidents, as these symptoms are often accompanied by a fall. If you notice any of the above symptoms, stop playing immediately. It is strongly recommended that parents supervise their children while playing video games, as children and adolescents are often more prone to photosensitive seizures than adults. If any such symptoms occur, STOP PLAYING IMMEDIATELY AND SEEK MEDICAL ADVICE. Parents and guardians should keep children in sight and ask them if they have ever experienced one or more of the above symptoms. Table of Contents System Requirements 3 Installation, Start, Uninstallation 4 Introduction: The Main Menu 5 In Game 6 HQ Placement / Contracts 6 Schedules 7 Cities / Clients 9 Trucks 10 Trailers / Truckshop / Truck fleets 11 Headquarters 12 Depots / Marketing 13 Rank / Concessions / Loans 14 Drivers / Upgrade Marketing / Competitors 15 Transport price levels 16 Keyboard Commands 17 Support 17 Credits 18 2 Welcome! Thank you for purchasing TransRoad: USA. In this manual, you will find useful tips that will allow you to enjoy the best gaming experience without problems.