ERUTEYA, Onoriode Christian A.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
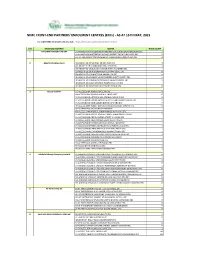
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

List of NIMASA Accredited Medical Providers
NIGERIAN MARITIME ADMINISTRATION AND SAFETY AGENCY SEARCH AND RESCUE BASE CLINIC MASTER LIST OF THE ACCREDITED SEAFARERS MEDICAL CERTIFYING HOSPITALS/CLINICS (UPDATED 2020) S/No Hospital/Clinic Name of Medical Director Allotted Code Address and Location GSM and Emailaddress WESTERN ZONAL REGISTER SAR BASE CLINIC APAPA WZL 000101 14, idewu Street, Olodi Apapa lagos [email protected] 1 Abbey Medical Dr. Otusanya O. A. WZL 000126 26A Pelewura Crescent Apapa 08033951195, 26A Pelewura Crescent 2 Adeiza Medical Centre Dr Peter Adeiza WZL 000125 A+B92:F92papa No 41, Cardoso Street, Kiri-Kiri 08050400776, 08093765811, 3 Asheco Hospital Dr. ISAH A. WZL 000114 [email protected] 2A KEFFI STREET IKOYI LAGOS 08029596408, 4 Bestcare Hospital Limited Dr. Bola Lawal WZL 000117 b/[email protected] 28, Randle Road, Apapa, Lagos 0803333031, [email protected], 5 Christ Medical Centre Ltd. Dr. P. I. Akinbodoye WZL 000127 [email protected] 37 Akinwunmi Street, joku Road, Sango Otta, Ogun 08036368730, 08023408686, 6 Faramed Clinic Dr. Farabiyi O. O. WZL 000106 State [email protected] 10 Alhaji kareem Akande Street, Off Sun Rise Bus Stop, 08033513638, 7 Grayma Medical Centre Dr. Ndukwe Emmanuel WZL 000102 Apapa - Oshodi Express Way Olodi Apapa [email protected], [email protected] 2 Nwabueze Close,Off Princess Aina Jegede Close, Ajao 08033270656 ,08037951190, 08033229546, Estate Lagos. [email protected] 8 Heda Hospital Dr. Ohaka Emma WZL 000104 1,Takoradi Road Apapa GRA, Lagos 08034020041, 08051186468, 9 Iduna Specialist Hospital Dr. UNUANE M. B WZL 000108 [email protected], [email protected] 11, ogunmodede street by Alade market, off Allen 08099726926, 08083126494, 10 Ikeja Medical Centre DR. -

Downloaded for Personal Non-Commercial Research Or Study, Without Prior Permission Or Charge
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] THE POLITICS AMO ADMINISTRATION OF COhTUNITY DEVELOPMENT IN THE RIVERS STATE OF NIGERIA BY LAURENCE A.8. lYAGOA Submitbed for the Degree of Doctor of Philosophy University of Glasgow Duly 1976 ProQuest Number: 10647271 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uesL ProQuest 10647271 Published by ProQuest LLO (2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLO. ProQuest LLO. -

Effect of N-Hexane Oil Extract of Two Spices on Serum Lipid Profile and Blood Glucose Concentration of Albino Rats by Ogunka-Nnoka C
Global Journal of Science Frontier Research Biological Science Volume 13 Issue 6 Version 1.0 Year 2013 Type : Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4626 & Print ISSN: 0975-5896 Effect of N-Hexane Oil Extract of Two Spices on Serum Lipid Profile and Blood Glucose Concentration of Albino Rats By Ogunka-Nnoka C. U. & Igwe F. U. Rivers State University of Science and Technology, Nigeria Abstract - Consumers are concerned with their health and physical fitness and are seeking for alternative plant products with potential for providing nutrients with enhanced health benefits. Hence, this study investigates the effect of mixture of Ehuru (Monodora myristica) and Njasang (Ricinodendron heudelotii) oil extract on serum lipid profile and blood glucose concentration of albino rats. The spices were processed into fine flour and the oil was extracted with n-hexane as the solvent. A total of twenty five rats weighing 125-160g were separated into five groups of five each to represent control, olive oil and varying concentrations of the spices. After acclimiatization for one week, experimental administration of the extract was carried out daily for 28 days. Blood samples were collected by cardiac puncture into tubes. A portion of the blood was used for fasting blood glucose determination. Serum was separated from the other portion and used for assay of lipid profile using standard kit methods. The results obtained showed percentage fatty acid yield for Ehuru and Njasang as 79.54 and 81.0 (polyunsaturated) and 13.40 and 15.0 (monounsaturated) respectively. Fasting blood glucose assay showed that only rats in group 1 (6.46mmol/L) became significantly (p<0.05) hyperglycaemic while groups 2-4(6.03, 5.98 and 5.53mmol/L) showed a hypoglycaemic effect with respect to control (6.13mmol/L). -

Articles in the Issue
Volume: 3 Issue: 4 ISSN: 2618 - 6578 BLACK SEA JOURNAL OF AGRICULTURE (BSJ AGRI) Black Sea Journal of Agriculture (BSJ Agri) is a double-blind peer-reviewed, open-access international journal published electronically 4 times (January, April, July and October) in a year since January 2018. It publishes, in English and Turkish, full-length original research articles, innovative papers, conference papers, reviews, mini-reviews, rapid communications or technical note on various aspects of agricultural science like agricultural economics, agricultural engineering, animal science, agronomy, including plant science, theoretical production ecology, horticulture, plant breeding, plant fertilization, plant protect and soil science, aquaculture, biological engineering, including genetic engineering and microbiology, environmental impacts of agriculture and forestry, food science, husbandry, irrigation and water management, land use, waste management etc. ISSN: 2618 - 6578 Phone: +90 362 408 25 15 Fax: +90 362 408 25 15 Email: [email protected] Web site: http://dergipark.gov.tr/bsagriculture Sort of publication: Periodically 4 times (January, April, July and October) in a year Publication date and place: October 01, 2020 - Samsun, TURKEY Publishing kind: Electronically OWNER Prof. Dr. Hasan ÖNDER DIRECTOR IN CHARGE Assoc. Prof. Uğur ŞEN EDITOR BOARDS EDITOR IN CHIEF Prof. Dr. Hasan ÖNDER Ondokuz Mayis University, TURKEY Assoc. Prof. Uğur ŞEN Ondokuz Mayis University, TURKEY SECTION EDITORS* Prof. Dr. Kürşat KORKMAZ, Ordu University, TURKEY Prof. Dr. Mehmet KURAN, Ondokuz Mayis University, TURKEY Prof. Dr. Muharrem ÖZCAN, Ondokuz Mayis University, TURKEY Prof. Dr. Mustafa ŞAHİN, Kahramanmaraş Sütçü İmam University, TURKEY Assoc. Prof. Dr. Esmeray Küley BOĞA, Cukurova University, TURKEY Assoc. Prof. Dr. Hasan Gökhan DOĞAN, Kirsehir Ahi Evran University, TURKEY Assoc. -

Download This Report
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION This document is important and should be read carefully. If you are in any doubt about its contents or the action to take, please consult your Stockbroker, Accountant, Banker, Solicitor or any other professional adviser for guidance immediately. For Information concerning certain risk factors which should be considered by Shareholders, see “Risk Factors” commencing on page 58. ACCESS BANK PLC RC 125384 RIGHTS ISSUE OF 7,627,639,636 ORDINARY SHARES OF N0.50 EACH AT N6.90 PER SHARE ON THE BASIS OF 1 (ONE) NEW ORDINARY SHARE FOR EVERY 3 (THREE) ORDINARY SHARES HELD AS AT 23 OCTOBER, 2014 PAYABLE IN FULL ON ACCEPTANCE ACCEPTANCE LIST OPENS: JANUARY 26, 2015 ACCEPTANCE LIST CLOSES: MARCH 04, 2015 ISSUING HOUSES LEAD ISSUING HOUSE RC 622258 JOINT ISSUING HOUSES RC 204920 RC 1031358 RC 685973 RC 189502 RC 160502 THE RIGHTS BEING OFFERED IN THIS CIRCULAR ARE TRADEABLE ON THE FLOOR OF THE NIGERIAN STOCK EXCHANGE FOR THE DURATION OF THE RIGHTS ISSUE. This Rights Circular and the Securities which it offers have been cleared and registered by the Securities & Exchange Commission. It is a civil wrong and a criminal offence under the Investments and Securities Act No. 29 2007 (the “Act”) to issue a Rights Circular which contains false or misleading information. Clearance and Registration of this Rights Circular and the Securities which it offers do not relieve the parties from any liability arising under the Act for false and misleading statements contained herein or for any omission of a material fact. -

**Wekpe V.O, Chukwu-Okeah, G.O & Godspower Kinikanwo Department
ROAD CONSTRUCTION AND TRACE HEAVY METALS IN ROADSIDE SOILS ALONG A MAJOR TAFFIC CORRIDOR IN AN EXPANDING METROPOLIS **Wekpe V.O, Chukwu-Okeah, G.O & Godspower Kinikanwo Department of Geography and Environmental Management, Faculty of Social Sciences, University of Port Harcourt, Rivers State, Nigeria. **Corresponding Author: [email protected] Abstract City growth often time results in advancement and development in transportation which comes with its attendant changes in road infrastructure and transport support services such as road side mechanic workshops, vulcanizers and bus stops. A byproduct of these attendant contiguous activities and processes is the emission and release of trace heavy metals. Trace heavy metals have been identified as major carcinogens. This study aimed at determining the occurrence and concentration of heavy metals in roadside soils in an expanding third world metropolis. To achieve the aim of the research, the total length of the road within the study section was measured. Ten sample locations were indentified at about 2.5km intervals along the road section under review. The heavy metal concentration was determined the using Buck Scientific 210 VGP Atomic Absorption Spectrophotometer. Heavy metals such as Iron (Fe), Copper (Cu), Cadmium (Cd), Lead (Pb) and Mercury (Hg) were determined. The result of the analysis showed that the concentration values ranged from <0.001 to 48.90 µg/mg. The results also revealed that the experimental sample points recorded higher values than the control samples; however, some of the control points had relatively higher concentration values. This observation may have emanated from the low lying trajectory and topography of the surrounding area, which allows run-off from the road side soils to wash off heavy metals and deposit them at these lower lying areas. -

Monodora Myristica) As a Flavourant in Cookie Production
International Journal of Food Studies IJFS October 2019 Volume 8 pages 1{12 Potentials of African Nutmeg (Monodora myristica) as a Flavourant in Cookie Production Kazeem K. Olatoyea*, Omololu O. Fapojuwoa, Joshu A. Olorunsholaa, and Julianah O. Ayorindea a Department of Food Science and Technology, College of Agriculture, Kwara State University, Malete, P.M.B 1530, Ilorin, Kwara State, Nigeria *Corresponding author [email protected] Received: 13 November 2017; Published online: 18 October 2019 Abstract African nutmeg, a possible local substitute for a commercial food flavourant, remains largely un- derutilized in Nigeria. Its application potential in cookie production was investigated in this study. African nutmeg (Monodora myristica) seed flour (ANM) was produced using a standard method. The flour was substituted for vanilla flavour (VFL) in ratio of 0, 1, 2, 3, and 3.5 g and functional proper- ties of the flour blends (water absorption capacity (WAC), oil absorption capacity (OAC), and bulk density) were determined, using standard methods. Cookies were developed and characterized chem- ically, physically (colour) and organoleptically using the AOAC method, a colourimeter and sensory panellists respectively. Data were analysed using ANOVA at p<0.05. Replacement of vanilla with African nutmeg had no significant effect on bulk density (0.62 g cm−3-0.68 g cm−3), but significantly affected WAC (133-142 %) and OAC (147-156 %) of flour blends. Crude protein (9.44-15.49 %), crude fat (3.17-6.50 %), total ash (2-2.73 %) and crude fibre (0.12-0.23 %) contents of the cookie increased, whilst metabolizable energy (385.33-367 kcal) decreased. -

Oxidative Stress Modulation by Cameroonian Spice Extracts in Hepg2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake
H OH metabolites OH Article Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake Achille Parfait Atchan Nwakiban 1 , Stefania Cicolari 2 , Stefano Piazza 2, Fabrizio Gelmini 3, Enrico Sangiovanni 2 , Giulia Martinelli 2 , Lorenzo Bossi 2, Eugénie Carpentier-Maguire 4, Armelle Deutou Tchamgoue 5, Gabriel A. Agbor 5 , Jules-Roger Kuiaté 1 , Giangiacomo Beretta 3 , Mario Dell’Agli 2,* and Paolo Magni 2,6,* 1 Department of Biochemistry, Faculty of Science, University of Dschang, P.O. Box 96 Dschang, Cameroon; [email protected] (A.P.A.N.); [email protected] (J.-R.K.) 2 Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Via G. Balzaretti 9, 20133 Milano, Italy; [email protected] (S.C.); [email protected] (S.P.); [email protected] (E.S.); [email protected] (G.M.); [email protected] (L.B.) 3 Department of Environmental Science and Policy, Università degli Studi di Milano, via G. Celoria 2, 20133 Milano, Italy; [email protected] (F.G.); [email protected] (G.B.) 4 Department of Science and Technology, University of Lille, Rue de Lille, 59160 Lille, France; [email protected] 5 Centre for Research on Medicinal Plants and Traditional Medicine, Institute of Medical Research and Medicinal Plants Studies (IMPM), Yaoundé 4124, Cameroon; [email protected] (A.D.T.); [email protected] (G.A.A.) 6 IRCCS MultiMedica, Sesto San Giovanni, Via Milanese, 300, 20099 Sesto San Giovanni Milan, Italy * Correspondence: [email protected] (M.D.); [email protected] (P.M.); Tel.: +39-0250318398 (M.D.); +39-0250318229 (P.M.) Received: 26 March 2020; Accepted: 29 April 2020; Published: 1 May 2020 Abstract: Oxidative stress plays a relevant role in the progression of chronic conditions, including cardiometabolic diseases. -

Seeds and Plants Imported
V? * •';' {."i'V i U. S. DEPARTMENT OF AGRICULTURE BOREAD OF PLANT INDUSTRY-BULLETIN NO. 132. B. T. GALLOWAY, Chief,of Bureau. SEEDS AND PLANTS IMPORTED DURING THE PERIOD FROM JULY, 1906, TO DECEMBER 31,1907: INVENTORY No. 13; Nos. 19058 TO 21730. ISSUED DECEMBER 4, 1908. WASHINGTON: GOVERNMENT PRINTING OFFICE. 190 8. BULLETINS OF THE BUREAU OF PLANT IJTOUSTRY. The scientific and technical publications of the Bureau of P.lant Industry, which was organized July 1, 1901, are issued in a single series of bulletins, a list of which follows. Attention is directed to the fact that the publications in this series are not for general distribution. The Superintendent ox Documents, Government Printing Office, Washington, D. cr, is authorised by law to sell them at cost, and to him all applications for these bulletins should be made, accompanied by a postal money order for the required amount or by cash. Numbers omitted from this list can not be furnished. No. 1. The Relation of Lime and Magnesia to Plant Growth. 1901. Price? 10 cents. 2. Spermatogenesis and Fecundation of Zamia. 1901, Price, 20 cents. 3. Macaroni Wheats. 1901. Price, 20 cents. 4.'Range Improvement in Arizona. 1901. Price, 10 cents. 6. A List of American Varieties of Peppers. 1902. Price, 10 cents. 7. The Algerian Durum Wheats. 1902. Price, 15 cents. 9. The North American Specie's'of Spartina. 1902. Price, 10 cents. 10. Records of Seed Distribution, etc. 1902. Price, 10 cents. 11. Johnson Grass. 1902. Price, 10 cents. , • 12. Stock Ranges of Northwestern California. 1902. Price, 15 cents. -

Does the Greater Port Harcourt Master Plan 2008 Meet Aspirations for Liveable City?
Ede et al., Greater Port Harcourt Master Plan 2008, 47th ISOCARP Congress 2011 Does the Greater Port Harcourt Master Plan 2008 meet Aspirations for Liveable City? By Precious N. Ede, Opuenebo B. Owei and Chimbiko Iche Akarolo In 2007 the government of Rivers State, Nigeria contracted a South African firm to produce a master plan for a new city called Greater Port Harcourt to be situated in the outskirts of the old city. The Greater Port Harcourt Master Plan 2008 is here examined in the context of current thinking as to whether it has credentials that meet aspirations for modern liveable cities. The new city plan assumes that Port Harcourt will continue to grow at its current rate so there is need to respond pro-actively to the reality of meeting cogent challenges. The infrastructure to be provided must be sustainable, that is, there should be continuity of effective service delivery by operators, by way of renewal, upgrading and expansion to cope with the city growth. Provision of services will be private sector driven, while government is politically ready to amend laws, regulations and policies to create an enabling environment for private sector to thrive in driving the development initiatives. The plan provides a long-term vision for the city based on sustainability: social, economic and political equity. Sustainability is hinged on continual improvement based on accountability, transparency and good governance. The master plan aims at a development that positively encourages the creation of a mixed community of 350,000 housing units, initially. The energy infrastructure utilizes the natural resources in the region such as natural gas for powering turbines and providing domestic fuel, with a little solar power. -

S/N COMPANY NAME ADDRESS LICENSE NUMBER 1 CVS Contracting International Ltd Suite 16B, Sabondale Shopping Complex, Jabi, Abuja CL/S&I/001/07
CLASS LICENCE REGISTER SALES AND INSTALLATIONS CATEGORY S/N COMPANY NAME ADDRESS LICENSE NUMBER 1 CVS Contracting International Ltd Suite 16B, Sabondale Shopping Complex, Jabi, Abuja CL/S&I/001/07 2 Telesciences Nig Ltd 123, Olojo Drive, Ojo Town, Lagos CL/S&I/002/07 3 Three One Three Communications Ltd No1, Isah Road, Badarawa, Kaduna CL/S&I/003/07 4 Latshak Global Concept Ltd No7, Abolakale Arikawe, ajah Lagos CL/S&I/004/07 5 Austin Willy Investment Ltd No 10, Willisco Street, Iju Ishaga Lagos CL/S&I/005/07 6 Geoinformatics Nig Ltd 65, Erhumwunse Street, Uzebu Qtrs, Benin City, Edo State CL/S&I/006/07 7 Dwellins Intl Ltd 21, Boyle Street, Onikan Lagos CL/S&I/007/07 8 Race Telecommunications Intl Ltd 19, Adebola Street, Surulere, Lagos CL/S&I/008/07 9 Clarfel Global Services Ltd Suite A45, Shakir Plaza, 3, Michika Strt, Off Ahmadu Bello Way, Area 11, Garki Abuja CL/S&I/009/07 10 MLD Temmy Concept Ltd FF1, Abeoukuta Street, Bida Road, Kaduna CL/S&I/010/07 11 King Chris Success Links Ltd No, 230, Association Shop, Old Epe Garage, Ijebu Ode, Ogun State CL/S&I/011/07 12 Diamond Sundries Ltd 54/56, Adeniji Street, Off Unity Street, Alakuko Lagos CL/S&I/012/07 13 Olucliff Nig Ltd Suite A33, Shakir Plaza, Michika Strt, Plot 1029, Area 11, Garki Abuja CL/S&I/013/07 14 Mecof Resources Ltd No 94, Minna Road, Suleja Niger State CL/S&I/014/07 15 Hypersand Communication Concept & Plot 29A, Democracy Crescent, Gaduwa Estate, Durumi 111, abuja CL/S&I/015/07 Solution Ltd 16 Patittas Nig Ltd Suite 17, Essence Plaza, Wuse Zone 6, Abuja CL/S&I/016/07 1 17 T.J.