Estimates of Inbreeding Rates in Ancient Belgian Chicken Breed Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SATURDAY 12Th JUNE 2021
SATURDAY 12th JUNE 2021 GRADED SHOW AND SALE OF 1304 LOTS COMPRISING OF RARE & PURE BREEDS OF POULTRY, BANTAMS, WATERFOWL, WILDFOWL, GEESE, TURKEYS, PEAFOWL, PHEASANTS, GUINEA FOWL, QUAIL, PIGEONS, DEADSTOCK, HATCHING EGGS AND BOXED YOUNG STOCK TO BE HELD AT FROME LIVESTOCK MARKET STANDERWICK, FROME, SOMERSET, BA11 2QB Tel: 01373 830033 SALE STARTS AT 9.00AM Catalogue £2.00 EVERYONE TO WEAR FACE MASK/COVERING AT ALL TIMES STRICT SECURITY ON SITE SALE TIMES 09.00am Deadstock, Hatching Eggs and Boxed Young Stock 10.00am ‘Championship Row’ 10.15am Large Fowl & Domestic Ducks followed by Guinea Fowl Ornamental Pheasants Quail Pigeons & Doves Ornamental Ducks Domestic Geese Turkeys Peafowl Ornamental Geese & Swans 10.30am Bantams (approx.) Gates open from 6.00am ALL birds to be penned by 9.00am ALL Deadstock to be in market field by 8.00am We are delighted with our entries and wish to thank all vendors for the huge variety of birds that you have to offer; it makes a particularly interesting catalogue. We urge all vendors and buyers attending the sale to use the hand washing/sanitation facilities provided. EVERYONE must supply their own face mask/covering which must be worn at all times. Anyone not complying with this rule will be asked to leave the premises. SOCIAL DISTANCING – We ask that you take this very seriously; this is not just for your safety but our staff too. We will be adhering to Government social distancing rules that are in place on the 12th June 2021. Please follow the instructions from market staff and security at all times. -

Danny Camerlinck
Nationale Kampioenschappen van België in Bastogne Gehouden 21 en 22 januari 2017 Tekst en foto’s: Danny Camerlinck Tijdens het weekend van 21 en 22 januari werden in het Waalse Bastogne de Belgische kampioenschappen gehouden. Er waren twee Regionale clubs die de handen in mekaar geslagen hadden, om de taak van de organisatie op zich te nemen. De locatie was de overdekte veemarkt waar normaal rundvee verhandeld werd. Helaas was er tijdens de periode van het gebeuren een heuse winterprik met temperaturen tot zelfs -15°C. Dit in combinatie met een heuse sneeuwstorm tijdens de voorbereiding, en de open structuur van het gebouw, zorgde voor een lastige editie van deze show. Anderzijds was België het enige land waar nog geen vogelgriep vastgesteld werd. Kort na de show was het wel zover, maar gelukkig hadden we toen reeds de kampioenschappen achter de rug. Er waren totaal een 2700 dieren ingeschreven en alles was mooi verdeeld over de beide lands- gedeelten en ook voor de soorten waren er bij alle diergroepen mooie aantallen. De Belgische Landsbond verzorgde de administratie, en er was ook een zéér rijk gevulde prijzentafel zodat er voor iedere deelnemer iets te rapen viel. We hadden dus een mooie show die zeker de moeite was om te bezoeken. De gezellige sfeer bij de vele aanwezigen was hiervan het bewijs. Een overzicht van vele raskampioenen in de fotoreportage hierna. During the weekend of 21 and 22 January, the Belgian championships were held in Bastogne, Wallonia. There were two regional clubs who had took on the organization of this event. The location was the market hall where were normally cattle is traded. -

Prijzen Van De Landsbond
Concours Nationaux – Nationale wedstrijden 2016-2017 I. PRIX DE LA FEDERATION NATIONALE PRIJZEN VAN DE LANDSBOND Secrétariat : Lars Van Droogenbroeck , Opgeëistenstraat 48 , 9470 Denderleeuw TEL : 0477 48 20 71 E-mail : [email protected] www.a-bassecour.be / www.neerhofdieren.be SUPER CHAMPIONS SECTION PIGEONS SUPERKAMPIOENEN SECTIE DUIVEN Cage Race Pred. Eleveur Hok Ras Fokker MEILLEUR PIGEON / BESTE DUIF 1616 Heurté de Saxe – Saksische snipduif 97/1 Verelst Louis MEILLEUR PIGEON RACE BELGE / BESTE DUIF BELGISCH RAS 1542 Culbutant belge – Belgische tuimelaar 97/1 Baert Henk Meuilleur pigeon de forme race belge / Beste vormduif Belgisch ras 1089 Voyageur belge d’expo – Belgische TT reisduif 97/1 De Vuyst William Meuilleur pigeon de forme race étrangère / Beste vormduif buitenlands ras 1150 Texan – Texan 96/1 Ars Henri Meilleur pigeon de structure / Beste struktuurduif 1309 Capucin – Raadsheer 97/1 Collin Kylian Meilleur culbutant ou haut-volant de race belge / Beste tuimelaar of hoogvlieger Belgisch ras 1503 Haut-volant belge – Belgische hoogvlieger 96/1 Pecheur Joseph Meilleur culbutant ou haut-volant de race étrangère / Beste tuimelaar of hoogvlieger buitenlands ras 1495 Culbutant de Cologne – Keulse tuimelaar 96/1 Baert Henk Meilleur boulant de race belge / Beste kropper Belgisch ras 1623 Boulant gantois – Gentse kropper 96/1 De Vetter Keano Meilleur boulant de race étrangère / Beste kropper buitenlands ras 1655 Boulant steller – Steller kropper 96/1 De Graeve Cindy Meilleur pigeon cravaté de race belge / Beste meeuwduif Belgisch -

The Flemish Cuckoo
THE FLEMISH CUCKOO Searching for their origin Text and photos: Norbert Schollaert (B) PART 3 CHICKEN BREEDS AND EXHIBITIONS From the middle of the 19th century, we see a shift towards greater consumption of imported products and again more meat. The advances in farming techniques by selecting and crossbreeding also followed out in the poultry world. But it was still the better middle class who could afford the keeping and breeding of purebred fowl. From this time, in America, Britain and the European mainland, distinguishable breeds of chicken got a breed name. The upper middle classes was developing an interest in keeping chickens for their ornamental value. The exhibitions of farmyard animals became popular. During the Belle Epoque, in 1900, the consumption of eggs doubled and the famous ‘poulets de Bruxelles’ were in great demand on the rich urban tables. The triangle Aalst - Mechelen - Brussels acquired fame with his fatteners which produced up to 1000 chickens per year. It was the heyday of the Belgian breeds, which locations we can see on a map from 1903 “Habitats des races Belges” (courtesy of the Image store of the CAG). In the north, the Campine; in the east the Braekel and Courtrai à cinq droits (The Courtrai or Kortrijk Fowl was a short-legged Braekel with 5 toes, that according to Edward Brown was a descendant of the Dorkings); in the south the Race Ardennaise and the Race Herve (the Ardenner and the Herve fowl) and in the centre a large region with Minorca (a Spanish variety with black plumes, which was seen in 1783 for the first time in England and in 1880 already was bred in Germany), the Race Coucou (The Cuckoo coloured chickens) and Brabançonnes. -

Le Grand Guide Des Poules Et Des Coqs 82 83 1
Sommaire AVANT-PROPOS .................................................................................................... 10 1 – Les coqs et poules sauvages ........................................................................... 12 Les différentes espèces ....................................................................................... 13 La mue d’été ...................................................................................................... 14 Les jeunes coqs .................................................................................................. 14 Le chant du coq ................................................................................................. 15 Habitudes à l’état sauvage ................................................................................... 16 Fiche 1 Les coqs Dorés .................................................................................... 16 Élevage ............................................................................................................. 17 Imitations .......................................................................................................... 17 Chasse du coq Doré ........................................................................................... 17 Menaces ............................................................................................................ 18 Coq Doré de Cochinchine ................................................................................. 18 Coq Doré de Birmanie ...................................................................................... -

Copyrighted Material
FTOC 08/27/2018 10:24:30 Page v Contents Acknowledgements ix Introduction 1 Standard feather markings 4 Chief points of the fowl 13 Complete classification of pure breed poultry 21 Defects and deformities 25 Large fowl and bantams 31 Ancona 31 Andalusian 34 Appenzeller 36 Araucana 41 Rumpless Araucana 45 Asil 48 Australorp 50 Autosexing breeds 53 Brockbar 54 Brussbar 55 Cambar 57 Dorbar 59 Legbar 60 Rhodebar 63 Welbar 65 Wybar 68 Ayam Cemani 71 Barnevelder 72 Belgian Bearded bantamsCOPYRIGHTED MATERIAL 75 Barbu d’Anvers 75 Barbu d’Uccle 77 Barbu de Watermael 78 Barbu d’Everberg (Rumpless d’Uccle) 80 Barbu du Grubbe (Rumpless d’Anvers) 81 Barbu de Boitsfort (Rumpless de Watermael) 81 Booted bantam 87 Rumpless Booted Bantam 92 Brabanter 93 Brahma 95 Brakel 100 FTOC 08/27/2018 10:24:30 Page vi vi Contents Breda 102 Bresse-Gauloise 104 Burmese 106 Campine 108 Cochin 111 Crèvecoeur 114 Croad Langshan 115 Dandarawi 118 Derbyshire Redcap 120 Dominique 121 Dorking 124 Dutch bantam 128 British Faverolles 133 Fayoumi 137 Friesian 139 Frizzle 143 German Langshan 145 Groninger 149 Hamburgh 152 Houdan 155 Indian Game 158 Ixworth 162 Japanese bantam 164 Jersey Giant 169 Ko Shamo 171 Kraienköppe 173 Kulang 177 La Flèche 179 Lakenvelder 181 Leghorn 183 Lincolnshire Buff 188 Malay 191 Marans 194 Marsh Daisy 198 Minorca 201 Modern Game 204 Modern Langshan 209 Nankin bantam 212 Nankin Shamo 214 New Hampshire Red 215 Norfolk Grey 218 North Holland Blue 220 Ohiki 222 Carlisle Old English Game 223 Oxford Old English Game 230 Old English Game bantam 236 Old English -

Poultry in the United Kingdom the Genetic Resources of the National Flocks
www.defra.gov.uk Poultry in the United Kingdom The Genetic Resources of the National Flocks November 2010 Cover: Red Dorking male (photograph John Ballard, courtesy of The Cobthorn Trust) All information contained in this brochure was correct at time of going to press (December 2010). Department for Environment, Food and Rural Affairs Nobel House 17 Smith Square London SW1P 3JR Telephone: 020 7238 6000 Website: www.defra.gov.uk © Crown copyright 2010 Copyright in the typographical arrangement and design rests with the Crown. This publication (excluding the logo) may be reproduced free of charge in any format or medium provided that it is reproduced accurately and not used in a misleading context. The material must be acknowledged as Crown copyright with the title and source of the publication specified. This document is also available on the Defra website. Published by the Department for Environment, Food and Rural Affairs. Printed in the UK, December 2010, on material that contains a minimum of 100% recycled fibre for uncoated paper and 75% recycled fibre for coated paper. PB13451 December 2010. Contents 1. Introduction 3 2. Poultry keeping systems 4 3. Species Accounts 6 3.1. The Domestic Fowl (Gallus gallus domesticus) 6 3.2. Turkeys 8 3.3. Ducks 8 3.4. Geese 9 3.5. Minor Species 9 4. Breed Organisations 10 5. Data Recording and Registration 11 6. References 12 7. Annex: Current situation for individual breeds and strains 13 Abbreviations 24 1 1. Introduction Domestic poultry form the most important sector of livestock keeping worldwide, the production of meat and eggs being a major contributor to human nutrition. -

NORMANDIE Annuaire Des Adhérents Et Leur Élevage En 2019
SOCIETE D’AVICULTURE de HAUTE –NORMANDIE Annuaire des adhérents et leur élevage en 2019 Volailles naines : Marans Volailles G.R. : Brahma AUGUSTIN Christian Ardennaise perdrix argenté 82170 MONBEQUI Lapins : Géant des Flandres BLONDEL François perdrix doré – perdrix bleu ! 05 63 27 71 91 blanc et gris garenne 76570 PAVILLY naines : Pékin argenté Pigeons : Strasser rouge ! 06 75 00 48 61 Brahma perdrix argenté Bouvreuil Archangel jaune Ayam Cemani BARBIER Frédéric Volailles naines : Chabo Sérama 77111 SOIGNOLLES blanche à queue noire en BRIE Chabo BOSCHAT Philippe Volailles G.R. : Marans ! 06 72 79 93 90 blanche à queue bleue 76810 GREUVILLE noir cuivré - bleu Pigeons : Texan BARRE Julien Canards :Mandarin - Carolin ( 02 35 04 15 17 Alouette de Cobourg 76190 S AINTE MARIE Pilet des Bahamas argenté ( 06 05 40 96 81 des CHAMPS _ Pilet d’Europe – Nette Rousse BOULIER Olivier Pigeons : Lynx de Pologne 02 35 56 87 56 Volailles naines : Hambourg 76740 HOUD ETOT _ bleu barré vol blanc argenté pailleté 02 35 57 05 36 Lynx de Pologne bleu Pékin porcelaine-Pékin blanc maillé poudré vol blanc BARREZ Christophe BOURDON Frédrick 76640 A LVIMARE Volailles G.R. : Marans NCC Volailles G.R. : Brahma ! 06 82 02 05 11 naines : Chabo blanche 76450 MALLEVILLE les GRES naines : Wyandotte à queue noire ( 07 80 40 44 48 BENARD Matthieu Pigeons : Calotte blanche 76190 SAINTE Oie Normande BOURJEOIS Martin d’Allemagne du Sud MARIE 76110 ECR AINVILLE pattes lisses bleu uni, bleu des CHAMPS Volailles : Gauloise dorée ! 07 68 57 35 73 écaillé, bleu barré, jaune ( 06 50 38 34 36 cendré écaillé, jaune Volailles G.R. -

British Poultry Standards
British Poultry Standards Complete specifi cations and judging points of all standardized breeds and varieties of poultry as compiled by the specialist Breed Clubs and recognised by the Poultry Club of Great Britain Sixth Edition Edited by Victoria Roberts BVSc MRCVS Honorary Veterinary Surgeon to the Poultry Club of Great Britain Council Member, Poultry Club of Great Britain This edition fi rst published 2008 © 2008 Poultry Club of Great Britain Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing programme has been merged with Wiley’s global Scientifi c, Technical, and Medical business to form Wiley-Blackwell. Registered offi ce John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom Editorial offi ce 9600 Garsington Road, Oxford, OX4 2DQ, United Kingdom For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. The right of the author to be identifi ed as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. -
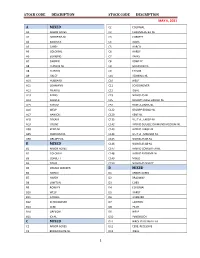
Stock Codes Numerical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C2 COLONIAL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 ANDREWS-NL C3 CORBETT A2 BABCOCK C4 DAVIS A3 CAREY C5 HARCO A5 COLONIAL C6 HARDY A6 EURIBRID C7 PARKS A7 GARBER C8 ROWLEY A8 H AND N-NL C9 GUILFORD-NL A8 H AND N C9 TATUM A9 HALEY C10 HENNING-NL A10 HUBBARD C10 WELP A11 LOHMANN C11 SCHOONOVER A12 MERRILL C12 IDEAL A13 PARKS C19 NICHOLAS-NL A14 SHAVER C35 ORLOPP LARGE BROAD-NL A15 TATUM C57 ROSE-A-LINDA-NL A16 WELP C122 ORLOPP BROAD-NL A17 HANSON C129 KENT-NL A18 DEKALB C135 B.U.T.A., LARGE-NL A19 HYLINE C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A38 KENT-NL C143 HYBRID LARGE-NL A45 MARCUM-NL C144 B.U.T.A., MEDIUM-NL A58 ORLOPP-NL C145 NICHOLAS 85-NL B MIXED C146 NICHOLAS 88-NL B1 ARBOR ACRES C147 HYBRID CONVERTER-NL B2 COLONIAL C148 HYBRID EXTREME-NL B3 CORBETT C149 MIXED B4 DAVIS C150 NICHOLAS SELECT B5 DEKALB WARREN D MIXED B6 HARCO D1 ARBOR ACRES B7 HARDY D2 BRADWAY B8 LAWTON D3 COBB B9 ROWLEY D4 COLONIAL B10 WELP D5 HARDY B11 CARGILL D6 HUBBARD B12 SCHOONOVER D7 LAWTON B13 CEBE D8 PILCH B14 OREGON D9 WELP B15 IDEAL D10 PENOBSCOT C MIXED D11 WROLSTAD SMALL-NL C1 ARBOR ACRES D11 CEBE, RECESSIVE C2 BROADWHITE-NL D12 IDEAL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N14 OLD ENGLISH, WHITE E MIXED N15 OLD ENGLISH, BLACK E1 COLONIAL N16 OLD ENGLISH, SPANGLED E2 HUBBARD N17 PIT E3 BOURBON, RED-NL N18 OLD ENGLISH E3 ROWLEY N19 MODERN E4 WELP N20 PIT, WHITE HACKLE E5 SCHOONOVER N21 SAM BIGHAM E6 CEBE N22 MCCLANHANS H MIXED N23 CLIPPERS H1 ARBOR ACRES N24 MINER BLUES H2 -

RARE POULTRY CLASSES 8 Malay Pullet
ASIAN HARDFEATHER 36 Ko Shamo Black/White/Self Pullet Regional Show 37 Ko Shamo AOC Male Sec: Z Shakeshaft Tel: 07866 575316 38 Ko Shamo AOC Female Judges : Mr R Francis (Large Fowl) 39 Ko Shamo AOC Stag & Mr P Cox ( Bantams) 40 Ko Shamo AOC Pullet 0 41 Asil Male LARGE FOWL 42 Asil Female 43 Tuzo Male 1 Asil Male 44 Tuzo Female 2 Asil Female 45 Non Standard/AOV Male 3 Asil Stag 46 Non Standard/AOV Female 4 Asil Pullet See Juvenile section for entries 5 Malay Male 6 Malay Female 7 Malay Stag RARE POULTRY CLASSES 8 Malay Pullet 9 O Shamo Male (8.8lbs+) RARE POULTRY SOCIETY 10 O Shamo Female (6.6lbs+) Club Show 11 Chu Shamo Male (Under 8.8lbs) Sec: P M Fieldhouse Tel: 01934 12 Chu Shamo Female (under 6.6lbs) 824213 (eve) 13 Shamo Stag (Any size) Judges: Mr J Lockwood 14 Shamo Pullet (Any Size) (Hardfeather & Soft Feather Heavy) 15 Kulang Male Mr P Hayford (True Bantams, 16 Kulang Female Longtails, Juveniles, Pairs & Trios) 17 Satsumadori Male Mr A Hillary (Soft Feather Light 18 Satsumadori Female Breeds 1) 19 Yamato Gunkei Male Mr J Robertson (Soft Feather Light 20 Yamato Gunkei Female Breeds 2) 21 Yamato Gunkei Stag 22 Yamato Gunkei Pullet LONG TAILED LARGE 23 Non Standard/AOV Male 24 Non Standard/AOV Female 47 Yokohama Male See Juvenile section for entries 48 Sumatra Male 49 Yokohama Female BANTAMS 50 Sumatra Female 25 Malay Male LARGE SOFTFEATHER LIGHT 26 Malay Female BREEDS 27 Ko Shamo Black Red Male 28 Ko Shamo Black Red Stag 51 Andalusian Male 29 Ko Shamo Duckwing Male 52 Andalusian Female 30 Ko Shamo Duckwing Stag 53 Ayam Cemani M/F -

David Scrivener Collection D DS
Museum of English Rural Life David Scrivener Collection D DS David Scrivener (1952 – 2015) was a committed and enthusiastic poultry- keeper. He earned a National Diploma in Poultry from Harper Adams Agricultural College in Shropshire. He utilised his expertise as a judge at various shows for the Poultry Club of Great Britain. David had a passion for rare breeds, particularly the Spanish, which he kept, and fulfilled various roles for the Rare Poultry Society, variously acting as Chairman, Honorary Historian and Patron. He was the author of several books on the subject including Starting with Bantams (2002), Exhibition Poultry Keeping (2005), Rare Poultry Breeds (2006) and Popular Poultry Breeds (2009). The Collection covers the year’s 1893 to 2007; undated. The physical extent of the collection is 17 boxes, 13 oversize items. D DS 1 Slides c. 1980 to 2015 Contains slides of various poultry species arranged by species and breed. Much of the information used here was obtained from Scrivener’s own annotations for each slide. Many of these photographs are studio portraits, used to illustrate Scrivener’s publications. A large proportion of the photographs were taken by John Tarren. Some boxes have been labelled with people’s names, presumably friends and fellow poultry breeders. 8 boxes Page 1 of 279 Museum of English Rural Life D DS 1/1 American Old English Game Box 1 c.1980 to 2015 Contains colour slides featuring photographs of various American Old English Game chickens. These images were all taken indoors, in some form of photographic studio. 6 slides D DS 1/1/1 Slide of a male American Old English Game chicken c.1980 to 2015 Studio photograph 1 slide D DS 1/1/2 Slide of an American Old English Game chicken c.1980 to 2015 Studio photograph, labelled AF.