The Transcription Factor Egr1 Is a Direct Regulator of Multiple Tumor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Growth Response 1 Acts As a Tumor Suppressor in Vivo and in Vitro Via Regulation of P53
Research Article Early Growth Response 1 Acts as a Tumor Suppressor In vivo and In vitro via Regulation of p53 Anja Krones-Herzig,1 Shalu Mittal,1 Kelly Yule,1 Hongyan Liang,2 Chris English,1 Rafael Urcis,1 Tarun Soni,1 Eileen D. Adamson,2 and Dan Mercola1,3 1Sidney Kimmel Cancer Center, San Diego, California and 2The Burnham Institute; 3Cancer Center, University of California at San Diego, La Jolla, California Abstract human tumor cell lines express little or no Egr1 in contrast to their normal counterparts (9–12). Furthermore, Egr1 is decreased or The early growth response 1 (Egr1) gene is a transcription factor that acts as both a tumor suppressor and a tumor undetectable in small cell lung tumors, human breast tumors promoter. Egr1-null mouse embryo fibroblasts bypass repli- (11, 13), and human gliomas (12). Reexpression of Egr1 in human tumor cells inhibits transformation. The mechanism of suppression cative senescence and exhibit a loss of DNA damage response h and an apparent immortal growth, suggesting loss of p53 involves the direct induction of TGF- 1 leading to an autocrine- functions. Stringent expression analysis revealed 266 tran- mediated suppression of transformation (8), increased fibronectin, scripts with >2-fold differential expression in Egr1-null mouse and plasminogen activator inhibitor (9). Egr1 also has been embryo fibroblasts, including 143 known genes. Of the 143 implicated in the regulation of p53 in human melanoma cells genes, program-assisted searching revealed 66 informative leading to apoptosis (14–16), and the proapoptotic tumor genes linked to Egr1. All 66 genes could be placed on a single suppressor gene PTEN also is directly regulated by Egr1 (17). -

A Dissertation Entitled the Androgen Receptor
A Dissertation entitled The Androgen Receptor as a Transcriptional Co-activator: Implications in the Growth and Progression of Prostate Cancer By Mesfin Gonit Submitted to the Graduate Faculty as partial fulfillment of the requirements for the PhD Degree in Biomedical science Dr. Manohar Ratnam, Committee Chair Dr. Lirim Shemshedini, Committee Member Dr. Robert Trumbly, Committee Member Dr. Edwin Sanchez, Committee Member Dr. Beata Lecka -Czernik, Committee Member Dr. Patricia R. Komuniecki, Dean College of Graduate Studies The University of Toledo August 2011 Copyright 2011, Mesfin Gonit This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of The Androgen Receptor as a Transcriptional Co-activator: Implications in the Growth and Progression of Prostate Cancer By Mesfin Gonit As partial fulfillment of the requirements for the PhD Degree in Biomedical science The University of Toledo August 2011 Prostate cancer depends on the androgen receptor (AR) for growth and survival even in the absence of androgen. In the classical models of gene activation by AR, ligand activated AR signals through binding to the androgen response elements (AREs) in the target gene promoter/enhancer. In the present study the role of AREs in the androgen- independent transcriptional signaling was investigated using LP50 cells, derived from parental LNCaP cells through extended passage in vitro. LP50 cells reflected the signature gene overexpression profile of advanced clinical prostate tumors. The growth of LP50 cells was profoundly dependent on nuclear localized AR but was independent of androgen. Nevertheless, in these cells AR was unable to bind to AREs in the absence of androgen. -

Egr1 (NM 007913) Mouse Tagged ORF Clone – MG227136 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for MG227136 Egr1 (NM_007913) Mouse Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: Egr1 (NM_007913) Mouse Tagged ORF Clone Tag: TurboGFP Symbol: Egr1 Synonyms: A530045N19Rik; egr; Egr-1; ETR103; Krox-1; Krox-24; Krox24; NGF1-A; NGFI-A; NGFIA; TIS8; Zenk; Zfp-6 Vector: pCMV6-AC-GFP (PS100010) E. coli Selection: Ampicillin (100 ug/mL) Cell Selection: Neomycin This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 4 Egr1 (NM_007913) Mouse Tagged ORF Clone – MG227136 ORF Nucleotide >MG227136 representing NM_007913 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) GACGTTGTATACGACTCCTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGGCGCGCCC ATGGCAGCGGCCAAGGCCGAGATGCAATTGATGTCTCCGCTGCAGATCTCTGACCCGTTCGGCTCCTTTC CTCACTCACCCACCATGGACAACTACCCCAAACTGGAGGAGATGATGCTGCTGAGCAACGGGGCTCCCCA GTTCCTCGGTGCTGCCGGAACCCCAGAGGGCAGCGGCGGTAATAGCAGCAGCAGCACCAGCAGCGGGGGC GGTGGTGGGGGCGGCAGCAACAGCGGCAGCAGCGCCTTCAATCCTCAAGGGGAGCCGAGCGAACAACCCT ATGAGCACCTGACCACAGAGTCCTTTTCTGACATCGCTCTGAATAATGAGAAGGCGATGGTGGAGACGAG TTATCCCAGCCAAACGACTCGGTTGCCTCCCATCACCTATACTGGCCGCTTCTCCCTGGAGCCCGCACCC AACAGTGGCAACACTTTGTGGCCTGAACCCCTTTTCAGCCTAGTCAGTGGCCTCGTGAGCATGACCAATC CTCCGACCTCTTCATCCTCGGCGCCTTCTCCAGCTGCTTCATCGTCTTCCTCTGCCTCCCAGAGCCCGCC -

Budesonide Epimer R Or Dexamethasone Selectively
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 3914–3919, March 1998 Medical Sciences Budesonide epimer R or dexamethasone selectively inhibit platelet-activating factor-induced or interleukin 1b-induced DNA binding activity of cis-acting transcription factors and cyclooxygenase-2 gene expression in human epidermal keratinocytes WALTER J. LUKIW*, RICARDO PALACIOS PELAEZ†,JORGE MARTINEZ†, AND NICOLAS G. BAZAN*‡ *Louisiana State University Medical Center, School of Medicine, Neuroscience Center of Excellence and Department of Ophthalmology, 2020 Gravier Street, Suite D, New Orleans, LA 70112-2272; and †Research and Development Department, IFIDESA-ARISTEGUI, Industrial Farmaceutica y de Especialidades SA, Maria de Molina 37, Madrid 28006 Spain Communicated by Ralph T. Holman, University of Minnesota, Austin, MN, January 2, 1998 (received for review July 23, 1997) ABSTRACT To further understand the molecular mecha- cytokines, matrix metalloproteinases, and cell adhesion molecules nism of glucocorticoid action on gene expression, DNA-binding known to be critical to both inflammation and the immune activities of the cis-acting transcription factors activator protein response (1, 2). Besides a direct “type 1” interaction with a 1 (AP1), AP2, Egr1 (zif268), NF-kB, the signal transducers and palindromic GC responsive element in GC-sensitive gene pro- activators of transcription proteins gamma interferon activation moters (3, 4), functions of the GC receptor also include a “type site (GAS), Sis-inducible element, and the TATA binding protein 2” interaction with chromatin-associated cis-acting transcription transcription factor II D (TFIID) were examined in human factors. For example, the GC receptor can physically interact with epidermal keratinocytes. The cytokine interleukin 1b (IL-1b) the Fos and Jun components of dimeric activator protein 1 (AP1) and platelet-activating factor (PAF), both potent mediators of (5, 6), the p65 subunit of NF-kB (7, 8), and the STAT5 family of inflammation, were used as triggers for gene expression. -
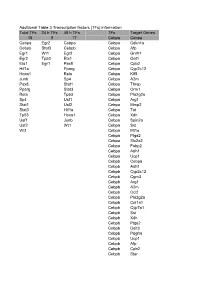
Additional Table 3 Transcription Factors (Tfs) Information Total Tfs
Additional Table 3 Transcription factors (TFs) information Total TFs 24 h TFs 48 h TFs TFs Target Genes 18 5 17 Cebpa Cebpa Cebpa Egr2 Cebpa Cebpa Cdkn1a Cebpb Stat3 Cebpb Cebpa Afp Egr1 Wt1 Egr2 Cebpa Gnrh1 Egr2 Tp53 Ets1 Cebpa Got1 Ets1 Egr1 Pax8 Cebpa Cpb2 Hif1a Pparg Cebpa Cyp2c12 Hoxa1 Rela Cebpa Klf5 Junb Sp4 Cebpa A2m Pax8 Stat1 Cebpa Thrsp Pparg Stat3 Cebpa Orm1 Rela Tp53 Cebpa Pla2g2a Sp4 Usf1 Cebpa Arg1 Stat1 Usf2 Cebpa Mmp2 Stat3 Hif1a Cebpa Tat Tp53 Hoxa1 Cebpa Xdh Usf1 Junb Cebpa Spin2a Usf2 Wt1 Cebpa Sst Wt1 Cebpa Mt1a Cebpa Ptgs2 Cebpa Slc2a2 Cebpa Fabp2 Cebpa Adh1 Cebpa Ucp1 Cebpb Cebpa Cebpb Adh1 Cebpb Cyp2c12 Cebpb Cgm3 Cebpb Arg1 Cebpb A2m Cebpb Ccl2 Cebpb Pla2g2a Cebpb Col1a1 Cebpb Cyp7a1 Cebpb Sst Cebpb Xdh Cebpb Ptgs2 Cebpb Ddit3 Cebpb Pdgfra Cebpb Ucp1 Cebpb Afp Cebpb Cpb2 Cebpb Star Cebpb Prl Cebpb Slc2a2 Egr1 Lhb Egr1 Adrb1 Egr1 Pgy1 Egr1 Th Egr1 Dlk1 Egr1 Chrna7 Egr1 Cntn1 Egr1 Slc9a3 Egr1 Pdgfb Egr1 Aldoc Egr1 Me1 Egr1 Pdgfa Egr1 Ptp4a1 Egr1 Serpine1 Egr1 Atp2a2 Egr1 Lhcgr Egr1 Mmp14 Egr1 Myh6 Egr1 Nudt6 Egr1 F3 Egr1 Id1 Egr2 Aldoc Ets1 Cited2 Ets1 Thy1 Ets1 Lhb Ets1 Runx2 Ets1 Ccnd1 Ets1 Spp1 Ets1 Casp3 Ets1 Prl Ets1 Mmp2 Ets1 Tat Ets1 Ucp1 Ets1 Ccnb1 Ets1 Cdkn1a Ets1 Mmp9 Hif1a Th Hif1a Wt1 Hif1a Vhl Hif1a Nos2 Hif1a Slc2a1 Hif1a Tfrc Hif1a Nppa Hif1a Serpine1 Hif1a Gck Hif1a Adra1a Hif1a Plat Hif1a Hmox1 Hif1a Mt1a Hif1a Pklr Hif1a Cdkn1a Hoxa1 Col1a1 Junb Th Junb Vgf Junb Timp1 Pax8 Wbp2 Pax8 Slc5a5 Pax8 Tg Pparg Hadha Pparg Ucp2 Pparg Aldh3a1 Pparg Nr1d1 Pparg Mapk1 Pparg Atp2a2 Pparg Ptgs2 Pparg Rxrg Pparg -

EGR1 Regulates Cellular Metabolism and Survival in Endocrine Resistant Breast Cancer
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 57), pp: 96865-96884 Research Paper EGR1 regulates cellular metabolism and survival in endocrine resistant breast cancer Ayesha N. Shajahan-Haq1, Simina M. Boca1,2,3,*, Lu Jin1,*, Krithika Bhuvaneshwar1,2,*, Yuriy Gusev1,2, Amrita K. Cheema1, Diane D. Demas1, Kristopher S. Raghavan1, Ryan Michalek4, Subha Madhavan1,2 and Robert Clarke1 1Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA 2Innovation Center for Biomedical Informatics (ICBI), Georgetown University Medical Center, Washington, DC, USA 3Department of Biostatistics, Bioinformatics and Biomathematics, Georgetown University, Washington, DC, USA 4Metabolon, Inc, Durham, NC, USA *These authors contributed equally to this work Correspondence to: Ayesha N. Shajahan-Haq, email: [email protected] Keywords: breast cancer, endocrine resistance, transcriptomics, metabolomics Received: October 26, 2016 Accepted: May 17, 2017 Published: May 30, 2017 Copyright: Shajahan-Haq et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT About 70% of all breast cancers are estrogen receptor alpha positive (ER+; ESR1). Many are treated with antiestrogens. Unfortunately, de novo and acquired resistance to antiestrogens is common but the underlying mechanisms remain unclear. Since growth of cancer cells is dependent on adequate energy and metabolites, the metabolomic profile of endocrine resistant breast cancers likely contains features that are deterministic of cell fate. Thus, we integrated data from metabolomic and transcriptomic analyses of ER+ MCF7-derived breast cancer cells that are antiestrogen sensitive (LCC1) or resistant (LCC9) that resulted in a gene-metabolite network associated with EGR1 (early growth response 1). -

The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders
REVIEW published: 06 March 2017 doi: 10.3389/fnbeh.2017.00035 The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders Florian Duclot 1,2 and Mohamed Kabbaj 1,2* 1Department of Biomedical Sciences, Florida State University, Tallahassee, FL, USA, 2Program in Neuroscience, Florida State University, Tallahassee, FL, USA It is now clearly established that complex interactions between genes and environment are involved in multiple aspects of neuropsychiatric disorders, from determining an individual’s vulnerability to onset, to influencing its response to therapeutic intervention. In this perspective, it appears crucial to better understand how the organism reacts to environmental stimuli and provide a coordinated and adapted response. In the central nervous system, neuronal plasticity and neurotransmission are among the major processes integrating such complex interactions between genes and environmental stimuli. In particular, immediate early genes (IEGs) are critical components of these interactions as they provide the molecular framework for a rapid and dynamic response to neuronal activity while opening the possibility for a lasting and sustained adaptation through regulation of the expression of a wide range of genes. As a result, IEGs have been tightly associated with neuronal activity as well as a variety of higher order processes within the central nervous system such as learning, memory and sensitivity to reward. The immediate early gene and transcription factor early growth response 1 (EGR1) has thus been revealed as a major mediator and regulator of synaptic plasticity and neuronal activity in both physiological and pathological conditions. In this review Edited by: article, we will focus on the role of EGR1 in the central nervous system. -

Novel Mutations Segregating with Complete Androgen Insensitivity Syndrome and Their Molecular Characteristics
International Journal of Molecular Sciences Article Novel Mutations Segregating with Complete Androgen Insensitivity Syndrome and Their Molecular Characteristics Agnieszka Malcher 1, Piotr Jedrzejczak 2, Tomasz Stokowy 3 , Soroosh Monem 1, Karolina Nowicka-Bauer 1, Agnieszka Zimna 1, Adam Czyzyk 4, Marzena Maciejewska-Jeske 4, Blazej Meczekalski 4, Katarzyna Bednarek-Rajewska 5, Aldona Wozniak 5, Natalia Rozwadowska 1 and Maciej Kurpisz 1,* 1 Institute of Human Genetics, Polish Academy of Sciences, 60-479 Poznan, Poland; [email protected] (A.M.); [email protected] (S.M.); [email protected] (K.N.-B.); [email protected] (A.Z.); [email protected] (N.R.) 2 Division of Infertility and Reproductive Endocrinology, Department of Gynecology, Obstetrics and Gynecological Oncology, Poznan University of Medical Sciences, 60-535 Poznan, Poland; [email protected] 3 Department of Clinical Science, University of Bergen, 5020 Bergen, Norway; [email protected] 4 Department of Gynecological Endocrinology, Poznan University of Medical Sciences, 60-535 Poznan, Poland; [email protected] (A.C.); [email protected] (M.M.-J.); [email protected] (B.M.) 5 Department of Clinical Pathology, Poznan University of Medical Sciences, 60-355 Poznan, Poland; [email protected] (K.B.-R.); [email protected] (A.W.) * Correspondence: [email protected]; Tel.: +48-61-6579-202 Received: 11 October 2019; Accepted: 29 October 2019; Published: 30 October 2019 Abstract: We analyzed three cases of Complete Androgen Insensitivity Syndrome (CAIS) and report three hitherto undisclosed causes of the disease. RNA-Seq, Real-timePCR, Western immunoblotting, and immunohistochemistry were performed with the aim of characterizing the disease-causing variants. -

Nobiletin Promotes Megakaryocytic Differentiation Through the MAPK/ERK-Dependent EGR1 Expression and Exerts Anti-Leukemic Effect
cells Article Nobiletin Promotes Megakaryocytic Differentiation through the MAPK/ERK-Dependent EGR1 Expression and Exerts Anti-Leukemic Effects in Human Chronic Myeloid Leukemia (CML) K562 Cells 1, 1, 1 2 Jui-Hung Yen y , Ching-Yen Lin y, Chin-Hsien Chuang , Hsien-Kuo Chin , Ming-Jiuan Wu 3 and Pei-Yi Chen 4,* 1 Department of Molecular Biology and Human Genetics, Tzu Chi University, Hualien 97004, Taiwan; [email protected] (J.-H.Y.); [email protected] (C.-Y.L.); [email protected] (C.-H.C.) 2 Division of Cardiovascular, Department of Surgery, Kaohsiung Armed Forces General Hospital, Kaohsiung 80284, Taiwan; [email protected] 3 Department of Biotechnology, Chia Nan University of Pharmacy and Science, Tainan 71710, Taiwan; [email protected] 4 Center of Medical Genetics, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien 97004, Taiwan * Correspondence: [email protected] or [email protected]; Tel.: +886-3-856-3092 These authors contributed equally. y Received: 15 February 2020; Accepted: 1 April 2020; Published: 3 April 2020 Abstract: Differentiation therapy is an alternative strategy used to induce the differentiation of blast cells toward mature cells and to inhibit tumor cell proliferation for cancer treatment. Nobiletin (NOB), a polymethoxyflavone phytochemical, is present abundantly in citrus peels and has been reported to possess anti-cancer activity. In this study, we investigated the anti-leukemic effects of NOB on cell differentiation and its underlying mechanisms in human chronic myeloid leukemia (CML) K562 cells. NOB (100 µM) treatment for 24 and 48 h significantly decreased viability of K562 cells to 54.4 5.3% and 46.2 9.9%, respectively. -

Molecular Changes Induced in Melanoma by Cell Culturing in 3D Alginate Hydrogels
cancers Article Molecular Changes Induced in Melanoma by Cell Culturing in 3D Alginate Hydrogels Melanie Kappelmann-Fenzl 1,†, Sonja K. Schmidt 2,†, Stefan Fischer 1, Rafael Schmid 3 , Lisa Lämmerhirt 2, Lena Fischer 4, Stefan Schrüfer 5, Ingo Thievessen 4, Dirk W. Schubert 5, Alexander Matthies 2, Rainer Detsch 6, Aldo R. Boccaccini 6 , Andreas Arkudas 3, Annika Kengelbach-Weigand 3 and Anja K. Bosserhoff 2,* 1 Faculty of Computer Science, Deggendorf Institute of Technology, Dieter-Görlitz-Platz 1, 94469 Deggendorf, Germany; [email protected] (M.K.-F.); stefan.fi[email protected] (S.F.) 2 Institute of Biochemistry, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Fahrstraße 17, 91054 Erlangen, Germany; [email protected] (S.K.S.); [email protected] (L.L.); [email protected] (A.M.) 3 Department of Plastic and Hand Surgery, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Krankenhausstraße 12, 91054 Erlangen, Germany; [email protected] (R.S.); [email protected] (A.A.); [email protected] (A.K.-W.) 4 Institute of Physics, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Henkestraße 91, 91054 Erlangen, Germany; lena.lf.fi[email protected] (L.F.); [email protected] (I.T.) 5 Institute of Polymer Materials, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Martensstraße 7, 91058 Erlangen, Germany; [email protected] (S.S.); [email protected] (D.W.S.) 6 Institute of Biomaterials, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Cauerstraße 6, 91058 Erlangen, Germany; [email protected] (R.D.); [email protected] (A.R.B.) Citation: Kappelmann-Fenzl, M.; * Correspondence: [email protected] Schmidt, S.K.; Fischer, S.; Schmid, R.; † These authors contributed equally to this paper. -

Systems Biology Analysis of Longitudinal Functional Response of Endothelial Cells to Shear Stress
Systems biology analysis of longitudinal functional response of endothelial cells to shear stress Nassim E. Ajamia, Shakti Guptab,c, Mano R. Mauryab,c, Phu Nguyenb,d, Julie Yi-Shuan Lib,d, John Y.-J. Shyye,1, Zhen Chene,f,1, Shu Chienb,d,e,1, and Shankar Subramaniama,b,d,g,h,1 aBioinformatics and Systems Biology Graduate Program, University of California at San Diego, La Jolla, CA 92023; bDepartment of Bioengineering, University of California at San Diego, La Jolla, CA 92093; cSan Diego Supercomputer Center, University of California at San Diego, La Jolla, CA 92093; dInstitute of Engineering in Medicine, University of California, San Diego, La Jolla, CA 92093; eDepartment of Medicine, University of California at San Diego, La Jolla, CA 92093; fDepartment of Diabetes Complications and Metabolism, Beckman Research Institute, City of Hope, CA 91010; gDepartment of Computer Science and Engineering, University of California, San Diego, La Jolla, CA 92093; and hDepartment of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, CA 92093 Contributed by Shu Chien, August 25, 2017 (sent for review May 8, 2017; reviewed by Michael A. Gimbrone, Jie Liang, and Jason A. Papin) Blood flow and vascular shear stress patterns play a significant role pulsatile flow, disturbed flow impairs endothelial homeostasis in inducing and modulating physiological responses of endothelial through three major processes: (i) It induces higher levels of re- cells (ECs). Pulsatile shear (PS) is associated with an atheroprotective active oxygen species (ROS) via the up-regulation of NADPH endothelial phenotype, while oscillatory shear (OS) is associated oxidase (NOX) and dysfunction of mitochondrial respiration chain with an atheroprone endothelial phenotype. -

Mutant P53 Initiates a Feedback Loop That Involves Egr-1&Sol;EGF
Oncogene (2010) 29, 2628–2637 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 $32.00 www.nature.com/onc ORIGINAL ARTICLE Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells L Sauer1, D Gitenay2, C Vo and VT Baron Vaccine Research Institute of San Diego, San Diego, CA, USA Early growth response-1 (Egr-1) is overexpressed in human to chemotherapy and radiation. The identification of key prostate tumors and contributes to cancer progression. On effectors of progression remains a crucial challenge. the other hand, mutation of p53 is associated with advanced Good evidence now supports the notion that early prostate cancer, as well as with metastasis and hormone growth response-1 (Egr-1) promotes the progression of independence. This study shows that in prostate cell lines in prostate cancer (reviewed in Gitenay and Baron, 2009). culture, Egr-1 overexpression correlated with an alteration Egr-1 is an early response transcription factor induced by a of p53 activity because of the expression of SV40 large T- wide range of growth factors and stress signals. The antigen or because of a mutation in the TP53 gene. In cells transcription of Egr-1 is increasedmostlybythemitogen- containing altered p53 activity, Egr-1 expression was activated protein-kinase (MAP-K) signaling pathway abolished by pharmacological inhibition or RNAi silencing through phosphorylation and activation of transcription of p53. Although forced expression of wild-type p53 was not factors of the Elk-1 family by ERK1/2, p38MAP-K and/ sufficient to trigger Egr-1 transcription, four different or JNK (reviewed in Silverman and Collins, 1999; Thiel mutants of p53 were shown to induce Egr-1.