A Translational Approach to Micro-Inflammation in End Stage Renal Disease: Molecular Effects of Low Levels of Interleukin 6
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Terza Categoria Salerno Gir
ANNO SPORTIVO 201 6/201 7 CAMPIONATO PROVINCIALE DI TERZA CATEGORA SALERNO - GIRONE " D" 1ª GIORNATA 2ª GIORNATA 3ª GIORNATA A. 13.11.2016 R. 19.02.2017 A. 20.11.2016 R. 26.02.2017 A. 27.11.2016 R. 05.03.2017 ARCI POSTIGLIONE BLACK LIONS ATLETI DI BACCO COLLIANO FUTURA ARCI POSTIGLIONE REAL BIANCA BUCCINESE REAL PERTOSA BLACK LIONS MONTEPRUNO ROSCIGNO ATLETI DI BACCO MAGORNO COLLIANO FUTURA MAGORNO MAGORNO REAL SAN GREGORIO BUCCINESE MONTESANGIACOMO MONTEPRUNO ROSCIGNO REAL OLIVETO CITRA MONTESANGIACOMO NADIR OLIVETO CITRA COLLIANO FUTURA REAL OLIVETO CITRA NADIR OLIVETO CITRA REAL BIANCA REAL BIANCA BUCCINESE MONTEPRUNO ROSCIGNO REAL PERTOSA REAL SAN GREGORIO MONTESANGIACOMO REAL PERTOSA ARCI POSTIGLIONE NADIR OLIVETO CITRA REAL SAN GREGORIO RIPOSA ATLETI DI BACCO RIPOSA REAL OLIVETO CITRA RIPOSA BLACK LIONS 4ª GIORNATA 5ª GIORNATA 6ª GIORNATA A. 04.12.2016 R. 12.03.2017 A. 11.12.2016 R. 19.03.2017 A. 18.12.2016 R. 26.03.2017 BLACK LIONS COLLIANO FUTURA ARCI POSTIGLIONE REAL SAN GREGORIO BLACK LIONS REAL OLIVETO CITRA MAGORNO NADIR OLIVETO CITRA ATLETI DI BACCO BLACK LIONS MAGORNO BUCCINESE MONTESANGIACOMO ARCI POSTIGLIONE BUCCINESE NADIR OLIVETO CITRA NADIR OLIVETO CITRA ARCI POSTIGLIONE REAL BIANCA MONTEPRUNO ROSCIGNO COLLIANO FUTURA REAL PERTOSA REAL BIANCA COLLIANO FUTURA REAL OLIVETO CITRA ATLETI DI BACCO MONTEPRUNO ROSCIGNO MONTESANGIACOMO REAL PERTOSA ATLETI DI BACCO REAL SAN GREGORIO BUCCINESE REAL OLIVETO CITRA MAGORNO REAL SAN GREGORIO MONTEPRUNO ROSCIGNO RISPOSA REAL PERTOSA RISPOSA REAL BIANCA RISPOSA MONTESANGIACOMO 7ª GIORNATA 8ª GIORNATA 9ª GIORNATA A. 08.01.2017 R. 02.04.2017 A. 15.01.2017 R. -

All'albo ISTITUTO COMPRENSIVO PELLEZZANO SITO WEB ATTI Prot. 2695/VII.1 Pellezzano, 22/09/2020 Oggetto: Personale Docente Pu
ISTITUTO COMPRENSIVO DI PELLEZZANO Sede legale: Via Nicola Russo, 7 84080 PELLEZZANO (SA) tel. 089/568134 e-mail [email protected] posta certificata: [email protected] C.F.95146400650 www.istitutocomprensivopellezzano.edu.it SAIC8BH007 All’ALBO ISTITUTO COMPRENSIVO PELLEZZANO SITO WEB ATTI Prot. 2695/VII.1 Pellezzano, 22/09/2020 Oggetto: Personale Docente Pubblicazione delle graduatorie provinciali per le supplenze G.P.S. II e III FASCIA VISTA l’O.M. n. 60 del 10 luglio 2020, concernente le procedure di istituzione delle graduatorie provinciali e di istituto di cui all’articolo 4, commi 6- bis e 6-ter, della legge 3 maggio 1999, n. 124 e di conferimento delle relative supplenze per il personale docente ed educativo; VISTO l’art. 8, comma 5°, della predetta O.M., secondo cui “gli uffici scolastici provinciali procedono alla valutazione dei titoli dichiarati per le GPS di competenza, anche attraverso la delega a scuole polo su specifiche classi di concorso, al fine di evitare difformità nelle valutazioni”; VISTO il decreto prot. n. 10324 del 20/08/2020, con cui venivano individuate le scuole polo relativamente alle materie di insegnamento/classi di concorso indicate negli allegati allo stesso decreto; VISTO il decreto prot. n. 13428 del 02/09/2020, con cui sono state pubblicate le GPS della provincia di Salerno per le quali risultavano effettuate le operazioni di validazione; DECRETA la pubblicazione, in data odierna delle graduatorie provinciali per le supplenze G.P.S. di II e III FASCIA del personale docente. Il Dirigente Scolastico F.to Dott.ssa Giovanna Nazzaro (Firma autografa sostituita a mezzo stampa ai sensi e per gli effetti dell’art.3. -

Ambito SA-23 Elenco Scuole Infanzia Ordinato Sulla Base Della Prossimità Tra Le Sedi Definita Dall’Ufficio Territoriale Competente
Anno Scolastico 2018-19 CAMPANIA AMBITO 0023 - DR Campania - Ambito SA-23 Elenco Scuole Infanzia Ordinato sulla base della prossimità tra le sedi definita dall’ufficio territoriale competente SEDE DI ORGANICO ESPRIMIBILE DAL Altri Plessi Denominazione altri Indirizzo altri Comune altri PERSONALE Scuole stesso plessi-scuole stesso plessi-scuole stesso plessi-scuole Codice Istituto Denominazione Istituto DOCENTE Denominazione Sede Caratteristica Indirizzo Sede Comune Sede Istituto Istituto Istituto stesso Istituto SAIC8BH007 IST.COMPR. PELLEZZANO SAAA8BH003 IST.COMPR. PELLEZZANO NORMALE VIA N.RUSSO, 7 PELLEZZANO SAAA8BH014 COLOGNA VIA G.MAZZINI PELLEZZANO SAAA8BH025 COPERCHIA VIA NICOLA RUSSO,7 PELLEZZANO SAAA8BH036 PELLEZZANO-CAPEZZAN VIA G.AMENDOLA PELLEZZANO O SAAA8BH047 SCUOLA INFANZIA DI PIAZZA G. DI VITTORIO, 1 PELLEZZANO PELLEZZANO SAAA8BH058 SCUOLA INFANZIA DI VIA SANTAMARIA AMATO, PELLEZZANO CAPRIGLIA 1 SAEE01400B BARONISSI SAAA014006 BARONISSI NORMALE VIALE SANDRO PERTINI 2 BARONISSI SAAA014017 BARONISSI CAPOLUOGO PARCO OLIMPIA BARONISSI SAAA014028 SCUOLA INFANZIA VIA FERRERIA BARONISSI CAPASIMO SAAA014039 AIELLO INFERIORE VIA SABATINO 11 BARONISSI SAAA01404A SCUOLA INFANZIA VIA S.ANDREA BARONISSI ANTESSANO SAAA01406C SARAGNANO VIA ARIOSTO BARONISSI SAAA01413R BARONISSI - "ORIGNANO" VIA BELLINI BARONISSI SAAA01414T BARONISSI"CAPRECANO VIA MENOTTI BARONISSI FUSARA" SAEE074003 MERCATO SAN SAAA07400T MERCATO SAN NORMALE VIA DEI DUE PRINCIPATI, MERCATO SAAA07402X E.PESCE VIA PIZZONE MERCATO SEVERINO I SEVERINO I 49/1 SAN SAN SEVERINO -

Curriculum Vitae Carlo Onnembo
CURRICULUM VITAE CARLO ONNEMBO Luogo e data di Battipaglia,17/12/1983 nascita Residenza Via Tempone delle Forche n. 35 , Postiglione, Salerno Domicilio Via Tempone delle Forche n. 35 , Postiglione (SA) E-Mail [email protected] ISTRUZIONE Febbraio 2016 19 ESIMO NELLA GRADUATORIA FINALE DI MERITO DEL QUINTO CORSO-CONCORSO SELETTIVO DI FORMAZIONE PER IL CONSEGUIMENTO DELL’ABILITAZIONE RICHIESTA AI FINI DELL’ISCRIZIONE DI N. 200 SEGRETARI COMUNALI NELLA FASCIA INIZIALE DELL’ALBO DEI SEGRETARI COMUNALI E PROVINCIALI, DENOMINATO “Co.A 5” Gennaio 2015 - Luglio 2015 Quinto Corso concorso di accesso alla carriera di Segretario Comunale ex art 13 d.p.r. 465/97 Presso la Scuola Superiore dell’Amministrazione dell’Interno Principali tematiche affrontate: L’azione amministrativa :Il procedimento amministrativo. Il Codice dei contratti pubblici (d. lgs. N. 163/2006) .Gli atti di gara e i sistemi di aggiudicazione La procedura ad evidenza pubblica Gli acquisti della P.A. La trasparenza amministrativa. I Servizi pubblici locali: elementi essenziali della normativa vigente - Le privatizzazioni e le liberalizzazioni - I servizi privi di rilevanza economica La potestà pianificatoria e l’assetto istituzionale delle competenze; gli strumenti urbanistici generali e attuativi. Il project financinig “spurio”; - Il confronto concorrenziale; - Lo scambio edificatorio. Vincoli urbanistici e città pubblica - Disciplina del prg e destinazione d’uso dei suoli; La SCIA - Il contributo di costruzione - L’abuso edilizio e le procedure sanzionatorie. L’espropriazione per pubblica utilità: fasi e presupposti. L’ordinamento contabile degli enti locali dopo l’armonizzazione, la struttura del bilancio e gli equilibri finanziari I principi contabili generali o postulati allegati al D.Lgs. -

Monti Alburni E La Valle Del Tanagro
LANDSCAPE AREA Q _ ALBURNI MOUNTAIN-TANAGRO VALLEY Included Geosites Id_denomination Imp. Id_denomination Imp. Id_denomination Imp. 142_ karsic springs with M 149_ Grooved mean- S 156_ karst plateau of S fall of S. Maria Auso ders of Calore river- Alburni Mountain Stazione di Sicignano 143_Caves of Castelcivita- F-C 150_Carbonatic series S 157_Piaggine Forma- S Mulino's springs of Postiglione tion-S. Rufo 144_Caves of Pertosa F-C 151_Terraces of Calore S 158_Fossil of Lady S river- Altavilla Silentina Mountain 145_Sinkhole of Fumo-Frà S 152_Limestone with S 159_ Richter side - S Gentile fossil-Timpa del Prato Controne 146_Fossiliferous level of S 153_Limestone with S 160_ Conglomerate of S Petina nummulites- Ottati Auletta 147_ Fault of Alburni S 154_ Trentinara forma- S Mountain -Il Figliolo tion-road to Castelcivita 148_Fossiliferous level of S 155_ Limestone with S Carbonara nummulites - road to Ottati M=Main F= Focal C= Complementary S= Secondary Legend Landscape areas National Park MAIN GEOSITE 142_ karsic springs with fall of S. Maria Auso Ubication Altitude Accessibility Fruition Nation - ITALY 300 m. s.l. Region - CAMPANIA feet trackway Province - SALERNO Distance car X recreation X NATIONAL PARK boat restaurant 70 Km. from Municipality - S. ANGE- National Park other exposition LO A FASANELLA office INTEREST (1= primary - 2 = secondary) GEOLOGICAL SCIENTIFIC OTHER Rare (conservation Structural Mineralogical experimentation) 1 Didactical 2 Stratigrafical Hydrogeological 1 Popularization Hikers/trecking Geomorphological 1 Paleontological 2 Rappresentative Archeological Sedimentological Karsic 1 Mondial/European 2 Naturalistic Paleoenviromental 2 Paleobiological National/Local Historical/religious Cartography of Main Geosite DESCRIPTION In this landscape area outcrop all the carbonate formations of Albuno Cervati Pollino Unit from the Tri- assic to the Middle Miocene. -

Monti Alburni
CLUB ALPINO ITALIANO Sez ione CAI Piedimonte Matese - Sezione CAI Napoli - Sezione CAI Foggia Monti Alburni - Monte Pizzuto (1403m) da Postiglione 29 Settembre 2019 Referenti: Antonio Del Giudice (CAI Piedimonte Matese) – Tania Pitta (CAI Piedimonte Matese) Giuliana Alessio (CAI Napoli) – Ester Guida (CAI Foggia) Il percorso L’itinerario proposto parte dalla piazza Europa nel centro abitato di Postiglione (a circa m 650 di quota) e, dirigendosi verso la chiesa principale, dopo circa 500 metri di stradina asfaltata, imbocca il sentiero n. 311 (dietro un muretto stradale, non segnato), che sale in un bosco misto dapprima alquanto pianeggiante in direzione Nord- NordOvest, attraversa due incisioni torrentizie, la seconda più marcata, in corrispondenza della quale la pendenza del fondo aumenta sensibilmente, ed inizia un lungo tratto a tornanti. Lungo questo tratto del sentiero si aprono alcuni affacci spettacol ari con splendido panorama sulle pareti rocciose degli Alburni e sulla valle del fiume Calore lucano, la piana del Sele, nonché sulle cime dei Monti Picentini. A partire dalla quota 1078 m, la direzione del sentiero piega decisamente a Sud Est, e la pendenza aumenta gradualmente, anche se il percorso a tornanti aiuta nella salita; si transita a tratti lungo pareti rocciose alte ed incombenti, spettacolari, ed il fondo diviene a sassi e terriccio: questa è la porzione del sentiero più delicata perché occorre non smuovere i sassi camminando! Alla fine della salita si valica facilmente il costone in una sella erbosa, il Varco Chiaimunno (1370 m) , qui il paesaggio cambia nettamente perché siamo giunti sull'altipiano degli Alburni dal quale spuntano le maggiori cime, quali La Nuda ed Il Figliolo , paesaggio caratterizzato da dolci ondulazioni del terreno, e a tratti bosco fitto, a tratti grandi radure erbose, con panorama mozzafiato sia sulle pareti rocciose verticali del massiccio degli Alburni , sia sulla valle sottostante col paese di Postiglione. -

Stemma-1443.2.Pdf
Un doveroso ringraziamento al dott. Generoso Conforti, all'Arci Postiglione e a quanti con questi hanno collaborato. Senza il loro enorme lavoro non ci sarebbe stato il nostro piccolo opuscolo. Prefazione Il simbolo più rappresentativo delle vicende che hanno afflitto la storia della Fasana, è lo scudo scolpito ai lati dell’altare al Cardoneto: vi è uno scudo bipartito, con il fagiano sulla destra, ma sulla sinistra un braccio che impone uno scudo più piccolo, al cui interno si intravede, per la cura del tempo, San Michele. Esprime il senso dell’imposizione, provata dalla comunità ottatese, per aver dovuto accettare lo stemma del compromesso santangiolese. Spiega perché non si trova in paese alcuna riproduzione di questo, mentre la nostra Fasana è dappertutto, tranne dove dovrebbe essere. Impone alle nostre coscienze di porre finalmente rimedio. Nel 1781, al momento della dedicazione della parrocchiale a San Biagio, monsignor Zuccari, vescovo di Capaccio, fece collocare una epigrafe che, tra l’altro, risolse la secolare questione circa la primogenitura di Fasanella, con la frase: ecclesiam hanc () Phasanellae primogenitam, cuius insignia, honorificentissima haec optatensium possidet universitas()1. Atteso che Sant’Angelo, nullius dioecesis, non apparteneva alla stessa diocesi, ed inoltre nel 1443 era già censito, assieme a Fasanella, fra le terre del Principato Citra, e Bellosguardo fu fondato dopo il 1266. Ottati, il casale più antico, esibisce la Fasana sui suoi monumenti più vetusti, la parrocchiale e il convento. Proprio il capitello della prima colonna, nel chiostro del convento, reca lo scudo con il fagiano: la fattura della Fasana, la storia del convento, creato ampliando la struttura di un antichissimo ospedale, la diversità dei materiali utilizzati per i due piani del chiostro, ci invitano ad ipotizzare che questa sia la riproduzione più antica della Fasana, risalente addirittura alla fondazione di Ott ati. -
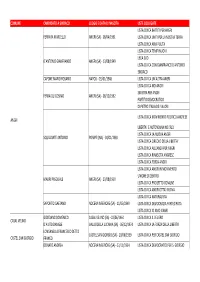
Comune Candidato a Sindaco Luogo E Data Di Nascita Liste Collegate
COMUNE CANDIDATO A SINDACO LUOGO E DATA DI NASCITA LISTE COLLEGATE LISTA CIVICA BATTITI PER ANGRI FERRARA MARCELLO ANGRI (SA) ‐ 08/04/1961 LISTA CIVICA UNITI PER LA NOSTRA TERRA LISTA CIVICA ARIA PULITA LISTA CIVICA TEMPI NUOVI LEGA SUD D'ANTONIO GIANFRANCO ANGRI (SA) ‐ 13/08/1949 LISTA CIVICA CON GIANFRANCO D'ANTONIO SINDACO CAPONE MARIO ROSARIO NAPOLI ‐ 25/01/1948 LISTA CIVICA UN'ALTRA ANGRI LISTA CIVICA NOI ANGRI SINISTRA PER ANGRI FERRAIOLI COSIMO ANGRI (SA) ‐ 08/12/1962 PARTITO DEMOCRATICO DI PIETRO ITALIA DEI VALORI LISTA CIVICA MOVIMENTO POLITICO ANGRESE ANGRI LIBERTA' E AUTONOMIA NOI SUD LISTA CIVICA LA NUOVA ANGRI SQUILLANTE ANTONIO POMPEI (NA) ‐ 04/01/1969 LISTA CIVICA CIRCOLO DELLA LIBERTA' LISTA CIVICA ALLEANZA PER ANGRI LISTA CIVICA RINASCITA ANGRESE LISTA CIVICA FORZA ANGRI LISTA CIVICA ANGRI IN MOVIMENTO UNIONE DI CENTRO MAURI PASQUALE ANGRI (SA) ‐ 23/08/1950 LISTA CIVICA PROGETTO COMUNE LISTA CIVICA ANGRI CITTA' NUOVA LISTA CIVICA NATURALISTA SAPORITO GAETANO NOCERA INFERIORE (SA) ‐ 11/05/1949 LISTA CIVICA DEMOCRAZIA PARTECIPATA LISTA CIVICA IO AMO ANGRI GIORDANO DOMENICO CASAL VELINO (SA) ‐ 03/06/1964 LISTA CIVICA IL VELIERO CASAL VELINO D'AIUTO DANIELE VALLO DELLA LUCANIA (SA) ‐ 06/12/1974 LISTA CIVICA LA FORZA DELLA LIBERTA' LONGANELLA FRANCESCO DETTO CASTEL SAN GIORGIO (SA) ‐ 20/06/1939 LISTA CIVICA PER CASTEL SAN GIORGIO CASTEL SAN GIORGIO FRANCO DONATO ANDREA NOCERA INFERIORE (SA) ‐ 23/11/1954 LISTA CIVICA DEMOCRATICI PER S. GIORGIO COMUNE CANDIDATO A SINDACO LUOGO E DATA DI NASCITA LISTE COLLEGATE LISTA CIVICA LA CAVA UNIONE DI CENTRO LISTA CIVICA CAVA PER LE LIBERTA' GALDI MARCO SALERNO ‐ 25/12/1965 MPA MOVIMENTO PER LE AUTONOMIE IL POPOLO DELLA LIBERTA' CAVA DE' TIRRENI LIBERTAS DEMOCRAZIA CRISTIANA MAZZEO MICHELE SALERNO ‐ 08/05/1946 RIFOND.COM. -

Roscigno Roscigno È Un Comune Italiano Di 675 Abitanti Della Provincia Di Salerno in Campania
1 Roscigno Roscigno è un comune italiano di 675 abitanti della provincia di Salerno in Campania. Roscigno fa parte della Comunità montana Alburni, del Parco nazionale del Cilento, Vallo di Diano e Alburni e della Diocesi di Teggiano - Policastro. Il nome del paese è una derivazione dalla dizione dialettale "russignuolo", vale a dire usignolo. Ha una economia agricola: abbondano sul suo territorio numerosi terreni coltivati ad olivi e viti. Roscigno Vecchia Il Comune di Roscigno si divide in due parti: Roscigno Vecchia e Roscigno Nuova. Roscigno Vecchia, il centro storico, è una frazione completamente disabitata da tempo a causa della presenza di diverse frane. Il centro storico di Roscigno inizia a svuotarsi intorno all'anno 1902 a causa di due ordinanze del genio civile (la legge speciale n. 301 del 7 luglio 1902 e la legge n. 445 del 9 luglio 1908) che obbligano la popolazione al trasferimento nell'attuale ubicazione del paese, Roscigno nuova. Storia Il nome del paese dalla ricostruzione storica deriva da Russino che compare in alcuni scritti del 1086 (custoditi presso l'Abbazia territoriale della Santissima Trinità di Cava de' Tirreni) quando il signore di Corneto (Corleto Monforte) donò la chiesa di S. Venere all'abbazia di Cava. Il piccolo borgo di Russigno e Ruscigni si può trovare menzionato in molti scritti di epoca medievale. Primi insediamenti abitativi I primi insediamenti abitativi, che costituirono uno dei primi nuclei abitati di Roscigno Vecchia, si formarono verso la fine dell'anno mille intorno ad un monastero di Benedettini che fu costruito ad un miglio a sud dalla località chiamata "Piano". -

Comune Di Postiglione (Sa)
COMUNE DI POSTIGLIONE (SA) PREMIO DI STUDIO "FRANCESCO ED EVELINA VECCHIO' La Associazione Culturale "Francesco ed Evelìna Vecchio" nell'ambito delle proprie attività' volte a favorire ed incoraggiare giovani studiosi meritevoli, bandisce un concorso per un premio di studio di mille euro, che sarà' assegnato alla tesi di laurea di qualunque materia che abbia come oggetto un argomento inerente al territorio del Cilento. La delimitazione territoriale del Cilento e' esplicitata nell'allegato al presente bando. La tesi deve essere stata discussa dopo un corso di Laurea magistrale o di Laurea a ciclo unico, in una delle sedi universitarie del territorio italiano o in una equivalente istituzione della Europa Unita nel periodo che va dal 1 giugno 2017 al 31 maggio 2018. I partecipanti al concorso debbono far pervenire una copia della tesi ed un certificato di laurea in formato digitale pdf entro il 30 giugno 2018 alla Associazione Culturale "Francesco ed Evelina Vecchio" [email protected] o all'indirizzo di posta certificata [email protected]. Tutti coloro che invieranno materiale per concorrere al presente bando, riceveranno via email conferma dell'avvenuto ricevimento. La valutazione delle tesi in concorso sarà' effettuata da una commissione presieduta dal presidente della Associazione e composta da altri studiosi che saranno nominati dal consiglio direttivo della Associazione, in funzione degli argomenti delle tesi in concorso. La Associazione si riserva il diritto di non assegnare il premio, qualora la commissione valuti inadeguati i lavori presentati. I risultati della valutazione e la data della premiazione saranno resi pubblici entro il 31 dì luglio del 2018. La premiazione si effettuerà' nel Comune di Ogliastro Cilento, Comune di nascita di Evelina Vecchio. -

S T a T U T O
S T A T U T O Approvato con delibera del Consiglio dei Delegati n. 13 del 19 maggio 2003 Approvato con deliberazione G.R. n. 0148/AC dell’11 luglio 2003 Modificato con delibera del Consiglio dei Delegati n. 2 dell’8 gennaio 2004 Approvato dalla Giunta Regionale della Campania con deliberazione n. 079/AC del 19 marzo 2004 Modificato con delibera del Consiglio dei Delegati n. 11 del 10 aprile 2008 Approvato dalla Giunta Regionale della Campania con decreto dirigenziale n. 162 del 29 maggio 2008 Modificato con deliberazione del Commissario Straordinario n. 21 del 22 giugno 2009 Approvato dalla Giunta Regionale della Campania con decreto dirigenziale n. 219 del 2 luglio 2009 Modificato con delibera del Consiglio dei Delegati n. 5 del 5 giugno 2015 Approvato dalla Giunta Regionale della Campania con decreto dirigenziale n. 50 del 16 luglio 2015 Modificato con deliberazione del Commissario Straordinario n. 20 del 01.02.2019 rettificata dalla deliberazione Commissariale n. 24 del 07.02.2019 Approvato dalla Giunta Regionale della Campania con decreto dirigenziale n. 25 del 20 marzo 2019 Modificato con deliberazione del Consiglio dei Delegati n. 6 del 25.06.2020 Approvato dalla Giunta Regionale della Campania con decreto dirigenziale n. 96 del 13 ottobre 2020 Capo 1° NATURA GIURIDICA – SEDE – COMPRENSORIO – PERIMETRO FUNZIONI - POTERI Art. 1 Natura Giuridica – Sede Il Consorzio bonifica di Paestum, costituito con Decreto Reale 25 Giugno 1926, registrato alla Corte dei Conti il 28 luglio 1926 al registro 12 – foglio 4685, ed ampliato con delibera della Giunta Regionale n. 9625 del 9/11/1979, è retto dal presente Statuto. -

Apri Allegato
Comune di Postiglione (Provincia di Salerno) Area Amministrativo/Finanziaria ORIGINALE di determina del Responsabile dell 'Area Amministrativo/Finanziaria COPIA N° 50/2018/Reg. Gen. OGGETTO: Impegno di spesa per adesione progetto "Condividere i bisogni per condividere il senso della vita" - anno 2018 dell' Associazione Banco alimentare Campania Onlus. L'anno duemiladiciotto, addì cinque, del mese di Febbraio , nella casa Comunale, il sottoscritto Assessore Comunale — Dott. Giovanni Costantino, Responsabile dell 'Area Amministrativo/Finananziaria, giusta provvedimento Sindacale nr. 2816/2017 e, quindi, abilitato a svolgere le funzioni di cui all'art. 107 del T.U. delle leggi sull'ordinamento degli Enti Locali, approvato con D. Lgs. 18 agosto 2000, n° 267; visto il vigente regolamento di contabilità; visto il vigente statuto comunale; visto il vigente regolamento comunale sull 'ordinamento degli uffici e dei servizi; visto il Decreto Legislativo nr. 267/2000; visto il Decreto Legislativo nr. 165/2001; vista, l'insusstenza di situazioni di incompatibilità e comunque delle situazioni di inconferibilità ed incompatibilità di cui all'art. 20 del D. Lgs. n. 39/2013; PREMESSO che il Comune nell'ambito della programmazione degli interventi socio assistenziali, sia propri, sia promossi e finanziati dal Piano di Zona S3 promuove azioni di contenimento della povertà e del bisogno contingente in favore di cittadini singoli o di nuclei familiari in condizione di difficoltà economica. CONSIDERATO che, in attuazione del proprio fine istituzionale di cura e gestione del territorio, previsto all'art. 3 del D. Lgs.vo n. 267/2000 e del principio di sussidiarietà orizzontale, sancito all'art. 118 della Costituzione, l'Amministrazione promuove ed incoraggia le iniziative dei cittadini, singoli o associati e con le realtà del terzo settore per lo svolgimento di attività di interesse generale e, per ciò che qui importa, per la realizzazione di azioni a sostegno delle persone bisognose.