Interaction and Transport of Methamphetamine and Its Primary Metabolites by Organic Cation and Multidrug and Toxin Extrusion Transporters S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mdma) in Non Conventional Matrices and Its Applications in Clinical Toxicology
Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY SIMONA PICHINI p Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY Doctoral thesis submitted by Simona Pichini as a partial fulfillment of the requirements for the degree of Doctor by the Universitat Autònoma de Barcelona. The studies included in this thesis have been realized under the direction of Dr. Rafael de la Torre Fornell and Dr. Magí Farré Albaladejo at the Pharmacology Unit of the Institut Municipal d'Investigació Mèdica (IMIM), Barcelona, Spain; and at the Drug Research and Control Department of the Istituto Superiore di Sanità, Roma, Italy. Doctorate Program of the Universitat Autònoma de Barcelona. Signature of Thesis Director Signature of Thesis Director (Dr. Magí Farré Albaladejo) (Dr. Rafael de la Torre Fornell) Signature of Doctorand (Simona Pichini) Yo soy yo y aquéllos a quienes amo Jorge Bucay To my friends, treasure of my life Acknowledgements Acknowledgements A number of people contributed to high extent to achieve the objectives of this Doctoral Thesis prepared between the two cities of Rome and Barcelona. This means that there are many people I’d the opportunity to meet, to work with, to share wonderful and terrible moments, and that I’d like to thank in these pages. First of all, to Dr. Piergiorgio Zuccaro, my “creator”, who always believed in me, supported my job and hardly fought to give me all the possible opportunities to grow up as an investigator; To Dr. -

The Excretion and Storage of Ammonia by the Aquatic Larva of Sialis Lutaria (Neuroptera)
THE EXCRETION AND STORAGE OF AMMONIA BY THE AQUATIC LARVA OF SIALIS LUTARIA (NEUROPTERA) BY B. W. STADDON* Department of Zoology, University of Durham, King's College, Newcastle upon Tyne (Received 12 April 1954) INTRODUCTION Delaunay (1931), in a review of the invertebrates, showed that an excellent correla- tion existed between the nature of the major nitrogenous component of the excreta and the nature of the environment, aquatic or terrestrial, in which an animal lived. Ammonia was shown to predominate in the excreta of aquatic species, urea or uric acid in the excreta of semi-terrestrial or terrestrial species. Delaunay put forward the view that the synthesis by these terrestrial forms of more complex molecules from ammonia was essentially a detoxication mechanism necessitated by a restricted water supply. Although the insects are primarily a terrestrial group, representatives of a number of orders have become aquatic in one or more stages of their life histories. It is well known that those terrestrial species which have been examined, with the notable exception of blowfly larvae, excrete the bulk of their nitrogen in the form of uric acid (Wigglesworth, 1950). The possibility, however, that aquatic species might have reverted to ammonotelism does not seem to have been examined. Preliminary tests were carried out on the excreta of a variety of aquatic insects. In all cases ammonia was found to be the major nitrogenous excretory product. An investigation into various aspects of the metabolism, toxicity and excretion of ammonia was then undertaken on the aquatic larva of Siatis lutaria. It is the purpose of the present communication to present some observations on the excretion and storage of ammonia in this species. -

In Vitro Interactions of Epacadostat and Its Major Metabolites With
DMD Fast Forward. Published on March 10, 2017 as DOI: 10.1124/dmd.116.074609 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 74609 In vitro interactions of epacadostat and its major metabolites with human efflux and uptake transporters: Implications for pharmacokinetics and drug interactions Qiang Zhang, Yan Zhang, Jason Boer, Jack G. Shi, Peidi Hu, Sharon Diamond, and Downloaded from Swamy Yeleswaram Incyte Corporation, Wilmington, DE 19803 dmd.aspetjournals.org at ASPET Journals on September 29, 2021 1 DMD Fast Forward. Published on March 10, 2017 as DOI: 10.1124/dmd.116.074609 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 74609 Running title: Interactions of Epacadostat with Transporters Corresponding Author: Qiang Zhang, Ph.D. Incyte Corporation 1801 Augustine Cut-Off Wilmington, DE 19803 Downloaded from Email: [email protected] Phone: 302-498-5827 Fax: 302-425-2759 dmd.aspetjournals.org Number of Text Pages: 24 Number of Figures: 8 at ASPET Journals on September 29, 2021 Number of Tables: 6 Number of References: 24 The number of words of Abstract: 247 The number of words of Introduction: 655 The number of words of Discussion: 1487 Non-standard Abbreviations: EPAC, epacadostat; IDO1, indoleamine 2,3-dioxygenase 1; EHC, enterohepatic circulation; NME, new chemical entity; TEER, transepithelial electrical resistance; HBSS, Hank's Balanced Salt Solution; KO, knockout; CSA, cyclosporin A; IC50, half-maximal inhibitory concentration; ER, efflux ratio 2 DMD Fast Forward. Published on March 10, 2017 as DOI: 10.1124/dmd.116.074609 This article has not been copyedited and formatted. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Poisons Act.Fm
LAWS OF BRUNEI CHAPTER 114 POISONS Enactment No. 13 of 1956 Amended by S 103/1958 Enactment No. 6 of 1967 S 101/1979 1984 Edition, Chapter 114 Amended by S 16/1996 S 28/2001 GN 273/2002 REVISED EDITION 2015 B.L.R.O. 1/2015 LAWS OF BRUNEI Poisons CAP. 114 1 LAWS OF BRUNEI REVISED EDITION 2015 CHAPTER 114 POISONS ARRANGEMENT OF SECTIONS Section 1. Citation. 2. Interpretation. 3. Description of poisons. 4. Licensing Officers. 5. General prohibition with respect to importation and sale of poisons. 6. Prohibitions and provisions relating to sale of poisons. 7. Exemptions in respect of medicines. 8. Exemptions in respect of sale. 9. Possession of poisons. 10. Issue of licences. 11. Different kinds of licence. 12. Licences to be numbered and registered. 13. Annual list to be published. 14. Forms of licence. 15. Search and search warrants. 16. Powers of exemption. 17. Penalties. B.L.R.O. 1/2015 LAWS OF BRUNEI 2 CAP. 114 Poisons 18. Jurisdiction. 19. Prosecutions. 20. Prohibition of sale to persons under 18. 21. Rules. SCHEDULE — POISONS LIST ____________________________ LAWS OF BRUNEI Poisons CAP. 114 3 POISONS ACT An Act to regulate the importation, possession, manufacture, compounding, storage, transport and sale of poisons Commencement: 1st January 1983 [S 61/1957] Citation. 1. This Act may be cited as the Poisons Act. Interpretation. 2. In this Act, unless the context otherwise requires — “dentist” means a dentist licensed under the Medical Practitioners and Dentists Act (Chapter 112) and includes a Government dentist; “export”, in relation -

TOXICOLOGY and EXPOSURE GUIDELINES ______(For Assistance, Please Contact EHS at (402) 472-4925, Or Visit Our Web Site At
(Revised 1/03) TOXICOLOGY AND EXPOSURE GUIDELINES ______________________________________________________________________ (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) "All substances are poisons; there is none which is not a poison. The right dose differentiates a poison and a remedy." This early observation concerning the toxicity of chemicals was made by Paracelsus (1493- 1541). The classic connotation of toxicology was "the science of poisons." Since that time, the science has expanded to encompass several disciplines. Toxicology is the study of the interaction between chemical agents and biological systems. While the subject of toxicology is quite complex, it is necessary to understand the basic concepts in order to make logical decisions concerning the protection of personnel from toxic injuries. Toxicity can be defined as the relative ability of a substance to cause adverse effects in living organisms. This "relative ability is dependent upon several conditions. As Paracelsus suggests, the quantity or the dose of the substance determines whether the effects of the chemical are toxic, nontoxic or beneficial. In addition to dose, other factors may also influence the toxicity of the compound such as the route of entry, duration and frequency of exposure, variations between different species (interspecies) and variations among members of the same species (intraspecies). To apply these principles to hazardous materials response, the routes by which chemicals enter the human body will be considered first. Knowledge of these routes will support the selection of personal protective equipment and the development of safety plans. The second section deals with dose-response relationships. -

Biotransformation: Basic Concepts (1)
Chapter 5 Absorption, Distribution, Metabolism, and Elimination of Toxics Biotransformation: Basic Concepts (1) • Renal excretion of chemicals Biotransformation: Basic Concepts (2) • Biological basis for xenobiotic metabolism: – To convert lipid-soluble, non-polar, non-excretable forms of chemicals to water-soluble, polar forms that are excretable in bile and urine. – The transformation process may take place as a result of the interaction of the toxic substance with enzymes found primarily in the cell endoplasmic reticulum, cytoplasm, and mitochondria. – The liver is the primary organ where biotransformation occurs. Biotransformation: Basic Concepts (3) Biotransformation: Basic Concepts (4) • Interaction with these enzymes may change the toxicant to either a less or a more toxic form. • Generally, biotransformation occurs in two phases. – Phase I involves catabolic reactions that break down the toxicant into various components. • Catabolic reactions include oxidation, reduction, and hydrolysis. – Oxidation occurs when a molecule combines with oxygen, loses hydrogen, or loses one or more electrons. – Reduction occurs when a molecule combines with hydrogen, loses oxygen, or gains one or more electrons. – Hydrolysis is the process in which a chemical compound is split into smaller molecules by reacting with water. • In most cases these reactions make the chemical less toxic, more water soluble, and easier to excrete. Biotransformation: Basic Concepts (5) – Phase II reactions involves the binding of molecules to either the original toxic molecule or the toxic molecule metabolite derived from the Phase I reactions. The final product is usually water soluble and, therefore, easier to excrete from the body. • Phase II reactions include glucuronidation, sulfation, acetylation, methylation, conjugation with glutathione, and conjugation with amino acids (such as glycine, taurine, and glutamic acid). -
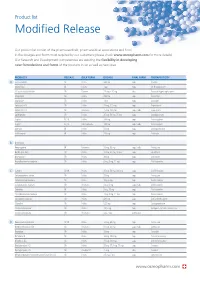
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Absorption, Distribution, Metabolism, Excretion and Toxicology Dr
Draft Guidance Novel Foods Absorption, Distribution, Metabolism, Excretion and Toxicology Dr. Josef Schlatter Member of the Scientific Committee and EFSA’s WG on Novel Foods Stakeholder Meeting, 11 April 2016, Brussels Section 7 Absorption, Distribution, Metabolism, and Excretion (ADME) 2 7. ADME (1) Critical for the development of appropriate toxicity testing strategy including the selection of appropriate animal models. Important information for the interpretation of study results. Differences between experimental animals and humans. Consider tiered approach to toxicokinetic testing which are described in section 4.1 on « Toxicokinetics (ADME) » of the EFSA Guidance for food additive evaluations (EFSA ANS Panel, 2012). Negligible absorption may provide a scientific justification for not undertaking higher tiered toxicological studies. Single substances and simple mixtures should normally be tested according to the same principles as those applied to food additives. As a default, absorption of the Novel Food or its breakdown products should be assessed. 3 7. ADME (2) For food additives in the form of complex mixtures, the ANS Guidance states that «conventional metabolism and toxicokinetic studies may not be feasible for all components in the mixture, but should be provided for toxicologically relevant constituents. Toxicologically relevant constituents are generally considered to be the major components and those other components with known or demonstrable biological or toxicological activity, and should be determined on a case-by-case basis with a scientific justification and the rationale for their selection provided ». Whole foods should be tested like complex mixtures as stated in the ANS Guidance. 4 7. ADME (3) For Novel Foods, ADME assessment should also address nutritionally significant constituents where toxicokinetic data on these constituents are important considerations for the evaluation of the nutritional impact of the Novel Food. -

Pharmacology Part 2: Introduction to Pharmacokinetics
J of Nuclear Medicine Technology, first published online May 3, 2018 as doi:10.2967/jnmt.117.199638 PHARMACOLOGY PART 2: INTRODUCTION TO PHARMACOKINETICS. Geoffrey M Currie Faculty of Science, Charles Sturt University, Wagga Wagga, Australia. Regis University, Boston, USA. Correspondence: Geoff Currie Faculty of Science Locked Bag 588 Charles Sturt University Wagga Wagga 2678 Australia Telephone: 02 69332822 Facsimile: 02 69332588 Email: [email protected] Foot line: Introduction to Pharmacokinetics 1 Abstract Pharmacology principles provide key understanding that underpins the clinical and research roles of nuclear medicine practitioners. This article is the second in a series of articles that aims to enhance the understanding of pharmacological principles relevant to nuclear medicine. This article will build on the introductory concepts, terminology and principles of pharmacodynamics explored in the first article in the series. Specifically, this article will focus on the basic principles associated with pharmacokinetics. Article 3 will outline pharmacology relevant to pharmaceutical interventions and adjunctive medications employed in general nuclear medicine, the fourth pharmacology relevant to pharmaceutical interventions and adjunctive medications employed in nuclear cardiology, the fifth the pharmacology related to contrast media associated with computed tomography (CT) and magnetic resonance imaging (MRI), and the final article will address drugs in the emergency trolley. 2 Introduction As previously outlined (1), pharmacology is the scientific study of the action and effects of drugs on living systems and the interaction of drugs with living systems (1-7). For general purposes, pharmacology is divided into pharmacodynamics and pharmacokinetics (Figure 1). The principle of pharmacokinetics is captured by philosophy of Paracelsus (medieval alchemist); “only the dose makes a thing not a poison” (1,8,9). -

Pdf 36.06 Kb
PROJECT REVIEW “Synthesis and Characterisation of Metabolites for the Integration in a Comprehensive Screening Procedure utilizing LC-MS/MS” W. Schänzer, M. Parr (German Sport University, Cologne, Germany) In the fight against doping the laboratories are confronted with an increasing number of substances to screen on. Therefore new methods have to be implemented by the laboratories. To keep the costs for doping control analysis acceptable, to ensure rapid reporting times and to lower the amount of urine needed to screen for all substances, a comprehensive screening for different classes of substances is desirable. Following the 2005 application WADA has granted a pilot project to check for the applicability of direct LC-MS/MS measurement of sulfoconjugates of heavy volatile stimulants. As most of the beta-2 agonists and heavy volatilestimulants are conjugated to sulfuric acid in humans an extension of the method to other compounds is desirable. As reference substances of the conjugates are barely available, during the pilot project the sulfoconjugates of p-Hydroxyamphetamine, p-Hydroxymetamphetamine (Pholedrine), p- Hydroxyephedrine, p-Hydroxynorephedrine, Etilefrine and Etamivan were synthesized by coupling the aglycons to sulfuric acid by reaction with sulfur trioxide pyridine complex. The objective of the continuation is to extend the combined screening procedure for diuretics and heavy volatile stimulants developed in the pilot project to other compounds. Thus, studies on the metabolism have to be reviewed and relevant metabolites have to be synthesised. The structures of all relevant products will be confirmed by nuclear magnetic resonance. For the integration in a comprehensive screening procedure the analytes will be characterised by means of LC-MS/MS. -

Isoxaflutole
ISOXAFLUTOLE First draft prepared by P.V. Shah 1 and Roland Alfred Solecki 2 1 Office of Pesticide Programs, Environmental Protection Agency, Washington, DC, United States of America (USA) 2 Federal Institute for Risk Assessment, Berlin, Germany Explanation ........................................................................................................................................... 393 Evaluation for acceptable daily intake .................................................................................................. 394 1. Biochemical aspects ............................................................................................................... 394 1.1 Absorption, distribution and excretion ............................................................................ 394 1.2 Biotransformation ........................................................................................................... 397 1.3 Dermal absorption ........................................................................................................... 399 2. Toxicological studies ............................................................................................................. 400 2.1 Acute toxicity .................................................................................................................. 400 (a) Oral administration .................................................................................................. 401 (b) Dermal application ..................................................................................................