2013 Wolf Lake Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Northwest Territories Territoires Du Nord-Ouest British Columbia
122° 121° 120° 119° 118° 117° 116° 115° 114° 113° 112° 111° 110° 109° n a Northwest Territories i d i Cr r eighton L. T e 126 erritoires du Nord-Oues Th t M urston L. h t n r a i u d o i Bea F tty L. r Hi l l s e on n 60° M 12 6 a r Bistcho Lake e i 12 h Thabach 4 d a Tsu Tue 196G t m a i 126 x r K'I Tue 196D i C Nare 196A e S )*+,-35 125 Charles M s Andre 123 e w Lake 225 e k Jack h Li Deze 196C f k is a Lake h Point 214 t 125 L a f r i L d e s v F Thebathi 196 n i 1 e B 24 l istcho R a l r 2 y e a a Tthe Jere Gh L Lake 2 2 aili 196B h 13 H . 124 1 C Tsu K'Adhe L s t Snake L. t Tue 196F o St.Agnes L. P 1 121 2 Tultue Lake Hokedhe Tue 196E 3 Conibear L. Collin Cornwall L 0 ll Lake 223 2 Lake 224 a 122 1 w n r o C 119 Robertson L. Colin Lake 121 59° 120 30th Mountains r Bas Caribou e e L 118 v ine i 120 R e v Burstall L. a 119 l Mer S 117 ryweather L. 119 Wood A 118 Buffalo Na Wylie L. m tional b e 116 Up P 118 r per Hay R ark of R iver 212 Canada iv e r Meander 117 5 River Amber Rive 1 Peace r 211 1 Point 222 117 M Wentzel L. -

Optimizing Alberta Parks Central Alberta
Optimizing Alberta Parks Central Alberta Legend Provincial Recreation Area 2020 Partial Closure (campground or Chrystina Chisholm Lesser Slave Lake Edith Lake Chain Lakes facility closure - remaining park areas will Shuttler Lake PRA Provincial PRA Chain Wolf Lake be open to public access, but Flats Big Mountain PRA Recreation Area Lakes PRA non-serviced) PRA Creek Central Peace-Notley PRA PRA Fort McMurray- Freeman 2020 Full Closure (entire site will be Kakwa Iosegun Lac La Biche Bonnyville- Grande Prairie-Wapiti River Pines River Trapper Lake Cold Lake- closed to public access) PRA PRA PRA PRA Lea's Cabin PRA Newbrook St. Paul Athabasca-Barrhead-Westlock PRA Proposed site partnership Muriel Southview Lake Mallaig Sheep PRA PRA Creek Smoke PRA PRA Lake Provincial Electoral Division * PRA Sheep Creek Morinville-St. Albert Kehiwin Smoky River Provincial Paddle River North National Park PRA South Recreation Area West Yellowhead Dam Bruderheim Kehiwin Population Centre PRA PRA Northwest of PRA Provincial Bruderheim Big Little Recreation Area Smoky River Berland Gunn Riverlot NA First Nation Sundance Creek PRA South Provincial PRA PRA Lac 56 Recreation Area Strathcona Metis Settlement Nojack Ste. Anne- NA Wildhay PRA Science Fort Saskatchewan-Vegreville PRA Parkland PP Military Base / Air Hornbeck Clifford E. Weapons Range Creek Lee Sherwood McLeod PRA NA Wildhorse Park Lake River * Due to space constraints, Provincial Weald NA PRA PRA Wolf Lake PRA Electoral Divisions within cities are not West Coal Lake Vermilion- PRA Edmonton labelled. Lovett North Lloydminster- Drayton Valley- PRA Watson River Wainwright PRA Brazeau Devon Creek Peaceful 0525 0 100 km Reservoir PRA Valley Pembina PRA PRA Maskwacis- Fairfax Lake Forks Elk Produced by Parks Division, Alberta Environment and Parks, Camrose PRA PRA River Wetaskiwin February 26, 2020. -

Occurrence and Source of Geogenic Arsenic in Groundwater from the Cold Lake-Beaver River Basin, Alberta Michael C
Occurrence and source of geogenic arsenic in groundwater from the Cold Lake-Beaver River Basin, Alberta Michael C. Moncur, S. Jean Birks, Emily Taylor, John J. Gibson Alberta Innovates-Technology Futures Dogan Paktunc CANMET Mining and Mineral Sciences Laboratory Brent Welsh Alberta Energy Regulator Carol J. Ptacek University of Waterloo Summary Elevated arsenic concentrations have been observed in shallow groundwater in the Cold Lake-Beaver River Basin (CLBR) of Alberta. The geology of this area includes up to 200 m of unconsolidated glacial deposits, with six regional interglacial sand and gravel aquifers, underlain by marine shale. Arsenic concentrations in unconsolidated sediment samples ranged between 1 to 17 ppm. Mineralogical characterization of the sediment samples revealed the presence of fresh framboidal pyrite in the deeper unweathered sediments with variable As contents of up to 1800 ppm. In contrast, the weathered sediments did not contain framboidal pyrite, but exhibited spheroidal Fe oxyhydroxide grains with elevated As concentrations, interpreted as pseudomorphs after pyrite. X-ray absorption near edge spectroscopy (XANES) indicated that the weathered sediments are dominated by As(V) species having spectral features similar to those of goethite or ferrihydrite with adsorbed As, suggesting that Fe oxyhydroxides are the dominant As carriers. XANES spectra collected from the unweathered sediment samples indicated the presence of a reduced As species characteristic of arsenopyrite and arsenian pyrite. A survey of over 800 water wells, isolated from industrial activity, were sampled for As and found that 50% of the wells contained As concentrations exceeding drinking water guidelines of 10 µg L-1. Higher As concentrations in groundwater were associated with increasing depth and reducing conditions, circumneutral pH and lower concentrations of SO4. -

Prepared For
Volume 5D, ESA – Trans Mountain Pipeline ULC Socio-Economic Technical Reports Trans Mountain Expansion Project Traditional Land and Resource Use Technical Report An Elder reported that he and fellow Ermineskin Cree Nation community members once fished for whitefish, pickerel, perch, rainbow trout, pike and bull trout in Wabamun Lake. However, due to an oil spill in 2005, the water quality is now poor and fishing is not ideal. The fish are small and are believed to be unhealthy due to pollution. Although Ermineskin Cree Nation community members do not travel to the lake to fish, community members from nearby bands still report it to be an important fishing site. Community members report that some of their past fishing sites are no longer used. An Elder identified Pigeon Lake as a fishing site (Plate 5.1.7-1). Most fishing takes place at the south end of the lake. Historically, net fishing has been conducted. Community members reported that Buck Lake was the best spot to catch whitefish in the past. Chimney Creek, near Kootenay Plains, was also a known fishing site, now used for grazing livestock and not often used by Ermineskin Cree Nation members. A cabin was once situated there. Plate 5.1.7-1 Pigeon Lake from helicopter overflight. TABLE 5.1.7-5 FISHING SITES IDENTIFIED BY ERMINESKIN CREE NATION Approximate Distance and Current/Past Requested Direction from Project Site Description Use Mitigation 31 km south of RK 15.4 Coal Lake Current None 51.8 km southwest of RK 29.9 Pigeon Lake Current None 24.6 km south of RK 61.4 Along North Saskatchewan River for Current None trout, sturgeon, rainbow trout, catfish, suckers and walleye. -
LAND OWNERSHIP MAP Map Produced by the Kilometers Geographic Information Systems Group ATS Township Privately Owned Land Lakes, Rivers Railway on April 15, 2020
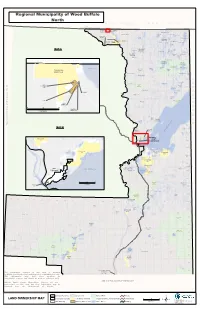
Regional Municipality of Wood Buffalo North NORTHWEST TERRITORIES THABACHA SEE 'MAP A' Selwyn Lake NARE 196A TSU TUE 196G Salt River 126 K'I TUE 196D Mercredi Treasure Loch Lake TTHE JERE Bayonet Lake Tulip GHAILI Dawson Lake Andrew Lake 196B FITZGERALD CHARLES Lake LAKE 225 LI DEZE 196C One Week Lake 125 THEBATHI 196 Arch Lake Charles Hutton Lake Lake Ashton Lake TSU K'ADHE Pans 124 MAP A Potts McLelland TUE 196F Lake Lake Lake Leland Lakes Leggo Lake Cherry Lake HOKEDHE Whaleback Lister Lake Lake TUE 196E St. Agnes 123 Myers Lake Lake Alexander Lake NORTHWEST TERRITORIES CORNWALL LAKE 224 COLLIN LAKE 223 Colin-Cornwall Cornwall Lakes Wildland Colin Lake Slave River Cockscomb Hooker Lake Lake Woodman THABACHA Lake Lake NARE 196A 121 Bocquene River Roderick Lake Peters Bocquene Lake Lake SASKATCHEWAN 120 De Manville Turtle Lake Lake La Butte Creek La Butte SLFN Creek Burstall DEVELOPMENT Wildland CORP. Lake 119 Wylie Lake Darwin Lake Bryant Lake SCHAEFER, C Slave River Florence Lake Barrow Lake Winnifred Lake FLINT, D Fidler-Greywillow Ryan Lake Wildland 474314 ALBERTA LTD 0 20 40 ROOTS & RUMINANTS INC. F O R Kilometers T S M I T H W I N T E Powder Creek R T RA IL Loutit Lake SANDY Flett POINT 221 a Lake c s Municipal District of Mackenzie No.23 a b a 114 h MAP B Riviere t des Rochers A ALLISON e DEVIL'S BAY 219 k GATE 220 a L 113 SEE 'MAP B' DEVIL'S GATE 220 FORT CHIPEWYAN 112 111 Goose Island ALLISON BAY Channel CHIPEWYAN 219 CHIPEWYAN 201B 201A Big Point Harrison Embarras Channel River Fletcher River Channel CHIPEWYAN 201 109 CHIPEWYAN Brander Lake Limon 201C OLD Fort Lake Old Fort Lake Athabasca FORT 217 Jackfish River Chipewyan Blanche Lake Richardson Creek 108 Lake CHIPEWYAN 201E F O R T . -

Summer Sport Fishery for Walleye and Northern Pike at Wolf Lake, Alberta, 2007
Summer Sport Fishery for Walleye and Northern Pike at Wolf Lake, Alberta, 2007 CONSERVATION REPORT SERIES The Alberta Conservation Association is a Delegated Administrative Organization under Alberta’s Wildlife Act. CCONSERVATIONONSERVATION RREPORTEPORT SSERIESERIES 25% Post Consumer Fibre When separated, both the binding and paper in this document are recyclable Summer Sport Fishery for Walleye and Northern Pike at Wolf Lake, Alberta, 2007 Bill Patterson Alberta Conservation Association #101, 9 Chippewa Road Sherwood Park, Alberta, Canada T8A 6J7 Report Editors PETER AKU KELLEY KISSNER Alberta Conservation Association 50 Tuscany Meadows Cres NW #101, 9 Chippewa Rd Calgary, AB T3L 2T9 Sherwood Park, AB T8A 6J7 Conservation Report Series Type Data ISBN printed: 978‐0‐7785‐7725‐6 ISBN online: 978‐0‐7785‐7726‐3 Publication No.: T/196 Disclaimer: This document is an independent report prepared by the Alberta Conservation Association. The authors are solely responsible for the interpretations of data and statements made within this report. Reproduction and Availability: This report and its contents may be reproduced in whole, or in part, provided that this title page is included with such reproduction and/or appropriate acknowledgements are provided to the authors and sponsors of this project. Suggested citation: Patterson, B. 2008. Summer sport fishery for walleye and northern pike at Wolf Lake, Alberta, 2007. Data Report, D‐2008‐004, produced by the Alberta Conservation Association, Sherwood Park, Alberta, Canada. 24 pp. + App. Cover photo credit: David Fairless Digital copies of conservation reports can be obtained from: Alberta Conservation Association #101, 9 Chippewa Rd Sherwood Park, AB T8A 6J7 Toll Free: 1‐877‐969‐9091 Tel: (780) 410‐1998 Fax: (780) 464‐0990 Email: info@ab‐conservation.com Website: www.ab‐conservation.com i EXECUTIVE SUMMARY The Alberta Conservation Association (ACA) conducted a creel survey at Wolf Lake during the 2007 summer angling season to provide information on angler use, sport fish yield and population structure. -

Mercury in Fish 2009-2013
2016 Mercury in Fish In Alberta Water Bodies 2009–2013 For more information on Fish Consumption Advisories Contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-0-7785- 8283-0 (Report) ISBN: 978-0-7785- 8284-7 (PDF) 2016 Government of Alberta Alberta Health, Health Protection Branch Mercury in Fish in Alberta Water Bodies 2009 – 2013 February 2016 Executive Summary Mercury enters the environment through various natural processes and human activities. Methylmercury is transformed from inorganic forms of mercury via methylation by micro-organisms in natural waters, and can accumulate in some fish. Humans are exposed to very low levels of mercury directly from the air, water and food. Fish consumers may be exposed to relatively higher levels of methylmercury by eating mercury-containing fish from local rivers and lakes. Methylmercury can accumulate in the human body over time. Because methylmercury is a known neurotoxin, it is necessary to limit human exposure. From 2009 to 2013, the Departments of Environment and Parks (AEP) and Health (AH) initiated a survey of mercury levels in fish in selected water bodies in Alberta. These water bodies are extensively accessed by the public for recreational activities. This report deals with (1) concentrations of total mercury levels in various fish species collected from the water bodies in Alberta, (2) estimated exposures, (3) fish consumption limits, (4) fish consumption advisories, and (5) health benefits of fish consumption. The results indicate that: 1. Concentrations of total mercury in fish in the water bodies in Alberta were within the ranges for the same fish species from other water bodies elsewhere in Canada and the United States. -

Beaver River Watershed Alliance
Beaver River Watershed Alliance About Us and Our Projects “The Little Watershed That Can…” March 15, 2011 Agri-Environmental Partnerships of Alberta AgForum, Nisku , AB Overview • BRiWthdBeaver River Watershed - GhGeography • Beaver River Watershed Alliance • BRWA Guiding Principles • BRWA Projects and Activities • Current BRWA Partnerships Beaver River Watershed - AB Beaver River Basin Cold Lake Edmonton Calgary Beaver River Watershed - Profile • Watershed area: ~ 22,000 km2 • Population: ~38,000 •Majjy(,)or urban centres: Bonnyville (5,600) and Cold Lake (12,000) • 4 counties, 4 Summer Villages, 1 MD, 1 Town, 1 City, 1 Village, 14 Hamlets • 3 Métis Settlements and 4 First Nation Reserves • Beaver River length: 491km (307 mi) • Originates from Beaver Lake near the town of Lac La Biche and extends eastward towar ds Sas kthkatchewan emp titying itinto the Hudson’s Bay at Churchill. • Average discharge: 653,000,000 m3/year (at AB/SK border) • Main tributaries: • Amisk (Little Beaver) River • 2000+ lakes including lakes within the Lakeland • Moose Lake River Provincial Recreation Area (e.g. Touchwood, • Sand River Pinehurst, Seibert, Spencer) while the lower part • Main economic activities include: includes lakes within the Cold Lake Air Weapons • Agriculture Range (e.g. Primrose Lake) • Oil and Gas • 12 Water Stewardship Groups, some with • Recreation • Society Status and others without Beaver River Watershed Alliance • The BRWA is a Watershed Planning and Advisory Council (WPAC) and an independent standing committee of the Lakeland Industry & Community Association (LICA) • Our main purpose is to develop an Integrated Watershed MtPlManagement Plan – This is accomplished in part by the completion of a State of the Watershed Report, which has been completed for the lower part of the basin – Cold Lake Beaver River State of the Watershed Report (()2006) • We, along with nine other provincial WPACs, are mandated by the Government of Alberta to carry out the three goals of Water for Life: Alberta ’ s Strategy for Sustainability (2003): 1. -

Surface Operations, Compliance, and Issues Not Related to Resource Evaluation and Recovery
SURFACE OPERATIONS, COMPLIANCE, AND ISSUES NOT RELATED TO RESOURCE EVALUATION AND RECOVERY February 6, 2019 PREMIUM VALUE. DEFINED GROWTH. INDEPENDENT. Outline - Surface Operations, Compliance, and Issues Not Related to Resource Evaluation and Recovery Page • Acronyms 4-5 • Facilities 6-7 ‒ Plot Plans, Simplified Plant Schematic, Modifications and Updates • Facility Performance 8-12 ‒ Oil & Water Treatment, Steam & Power Generation, Gas Usage, Greenhouse Gas Emissions • Measurement and Reporting 13-16 ‒ Well Production Estimates, Proration factors, Test Durations, New Measurement Technology • Water Production, Injection and Uses 17-26 ‒ UWIs, Water Uses and Water Quality ‒ Fresh, Brackish, Steam and Produced Water Volumes & Forecasts ‒ Brackish Water Supply ‒ Directive 081 Update • Disposal and Waste 27-37 ‒ UWIs & Disposal Well Compliance ‒ Wolf Lake Disposal & Water Storage Volumes ‒ Wolf Lake Waste Disposal ‒ Wastewater and Surface Runoff Slide 2 Outline - Surface Operations, Compliance, and Issues Not Related to Resource Evaluation and Recovery Page • Sulphur Production 38-40 • Environmental Summary 41-53 ‒ Compliance Issues & Amendments ‒ Monitoring Programs ‒ Reclamation ‒ Regional Initiatives ‒ Groundwater Monitoring • Approval Condition Compliance 54-62 ‒ Approvals (9140QQ, 9108, 8186A, 8672E, 8673, 3929A, 4128D, 9792A) • Compliance 63 • Future Plans 64 Slide 3 Primrose, Wolf Lake, and Burnt Lake Annual Directive 54 Presentation AER Alberta Energy Regulator EPEA Alberta Environmental Protection and Enhancement Act ALMS Alberta -

Physical Features
Physical Features Chapter 2 Prepared by J. Brad Stelfox, John Godfrey, Mike Flannigan, Bob Wynes, and Greg Tink Summary Points • The geological landscape of northwest Alberta reflects the dynamic erosional and depositional history of the region. Advancing and retreating glaciers during the Pleistocene modified the pre-glacial topography, smoothing the highlands and depositing materials in topographic depressions. • Today, morainal deposits blanket most of the lowland regions. Glacial deposits on upland areas are more shallow and consequently these regions have some Cretaceous and Tertiary sediments exposed. • Soil conditions in northwest Alberta vary significantly depending on glacial history, local parent materials, climate, vegetation, and topography, with finer-textured clay-dominated soils occurring in glacial lake basins, and clay/loams with some stoniness occurring in highland regions where glacial till prevails. • Permafrost is present throughout much of the northern portions of the study area and is generally found beneath bog complexes. Permafrost is common in more northerly latitudes but yields to degraded permafrost further south. Degraded permafrost areas are those sites that provide clear evidence of permafrost in recent geologic times, but has been degraded because of changes in climate or landform. • The overall climate for northwest Alberta is continental, with warm summers and cold winters. Large contrasts in temperature between day and night, and between winter and summer are typical. For seven to eight months of the year, the air transport in northwest Alberta is from the west, which might be expected to bring moist air from the Pacific. However, most of the moisture is lost when the air passes over the Coastal Mountain Range and the Rocky Mountains, thereby giving the region its continental climate. -

The Influence of the Pacific Decadal Oscillation on Annual Floods in the Rivers of Western Canada1
JOURNAL OF THE AMERICAN WATER RESOURCES ASSOCIATION AMERICAN WATER RESOURCES ASSOCIATION THE INFLUENCE OF THE PACIFIC DECADAL OSCILLATION ON ANNUAL FLOODS IN THE RIVERS OF WESTERN CANADA1 Sunil Gurrapu, Jeannine-Marie St-Jacques, David J. Sauchyn, and Kyle R. Hodder2 ABSTRACT: We analyzed annual peak flow series from 127 naturally flowing or naturalized streamflow gauges across western Canada to examine the impact of the Pacific Decadal Oscillation (PDO) on annual flood risk, which has been previously unexamined in detail. Using Spearman’s rank correlation q and permutation tests on quantile-quantile plots, we show that higher magnitude floods are more likely during the negative phase of the PDO than during the positive phase (shown at 38% of the stations by Spearman’s rank correlations and at 51% of the stations according to the permutation tests). Flood frequency analysis (FFA) stratified according to PDO phase suggests that higher magnitude floods may also occur more frequently during the negative PDO phase than during the positive phase. Our results hold throughout much of this region, with the upper Fraser River Basin, the Columbia River Basin, and the North Saskatchewan River Basin particularly subject to this effect. Our results add to other researchers’ work questioning the wholesale validity of the key assumption in FFA that the annual peak flow series at a site is independently and identically distributed. Hence, knowledge of large- scale climate state should be considered prior to the design and construction of infrastructure. (KEY TERMS: Western Canada; floods; independently and identically distributed assumption (i.i.d.) of flood fre- quency analysis; multi-decadal variability; Pacific Decadal Oscillation; Fraser River Basin; Columbia River Basin; North Saskatchewan River Basin; permutation test for quantile-quantile plots.) Gurrapu, Sunil, Jeannine-Marie St-Jacques, David J. -

Prepared For: Prepared By
NOVA Gas Transmission Ltd. Environmental and Socio-Economic Assessment Liege Lateral Loop 2 – Thornbury Section Pipeline and September 2014 / 496955/492475 the Leismer East Compressor Station Project APPENDIX 10 TRADITIONAL KNOWLEDGE REPORT Page A10-1 TRADITIONAL KNOWLEDGE REPORT FOR THE PROPOSED LIEGE LATERAL LOOP 2 – THORNBURY SECTION PIPELINE AND THE LEISMER EAST COMPRESSOR STATION PROJECT FOR THE COMMUNITIES OF: BEAVER LAKE CREE NATION BIGSTONE CREE NATION CHIPEWYAN PRAIRIE DENE FIRST NATION CHRISTINA RIVER DENE NATION COUNCIL CONKLIN MÉTIS LOCAL No. 193 FORT MCMURRAY FIRST NATION No. 468 FORT MCMURRAY MÉTIS LOCAL No. 1935 FORT MCMURRAY MÉTIS LOCAL No. 2020 HEART LAKE FIRST NATION LAKELAND MÉTIS LOCAL No. 1909 MÉTIS NATION OF ALBERTA MÉTIS NATION OF ALBERTA ZONE 1 OWL RIVER MÉTIS LOCAL No. 1949 SADDLE LAKE CREE NATION WILLOW LAKE MÉTIS LOCAL No. 780 September 2014 496955/492475 THE CONFIDENTIALITY OF THE LOCATION AND NATURE OF USE OF ALL SITES HAS BEEN PRESERVED THROUGHOUT THIS REPORT Prepared for: Prepared by: NOVA Gas Transmission Ltd. CH2M HILL Energy Canada, Ltd. A Wholly Owned Subsidiary of TransCanada PipeLines Limited Suite 1100, 815 - 8th Avenue S.W. Calgary, Alberta T2P 3P2 Calgary, Alberta Ph: 403-265-2885 NOVA Gas Transmission Ltd. Traditional Knowledge Report Liege Lateral Loop 2 – Thornbury Section Pipeline and September 2014 / 496955/492475 the Leismer East Compressor Station Project EXECUTIVE SUMMARY NOVA Gas Transmission Ltd. (NGTL), a wholly owned subsidiary of TransCanada PipeLines Limited is proposing to construct and operate the Liege Lateral Loop 2 – Thornbury Section Pipeline and the Leismer East Compressor Station (the Project). The Project will be part of the NGTL System, a network of 24,400 km of pipeline that transports natural gas for use within Alberta and British Columbia and for delivery to other markets across North America.