Susceptibility to Size Visual Illusions in a Non-Primate Mammal (Equus Caballus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tesi Olga.Pdf
CONTENTS Preface III Introduction 1 Visual illusions as research tools in perception 1 The Delboeuf size-contrast illusion 4 Historical sketch 5 A new effect observed within modified Delboeuf size-contrast displays 6 Lightness: terminology and research issues 7 The object of the study and hypotheses of the study 9 Chapter I. Experiment 1: Brigner vs Zanuttini and Daneyko 11 1.1 Experiment 1 11 1.2 Discussion 15 Chapter II. Testing the role played by the luminance values of the size-contrast inducers 17 2.1 Experiment 2 20 2.2 Discussion 22 2.3 Experiment 3: is the size-contrast effect still there? 23 2.4 General discussion 25 Chapter III. Testing the role played by perceived depth 27 3.1 Experiment 3: which target appears closer? 28 3.2 Experiment 4: lightness and depth in stereoscopic displays 30 3.3 Discussing the results with reference to lightness theories 33 3.4 Lightness, depth, and belongingness 38 Chapter IV. Belongingness or size? 41 I 4.1 Experiment 5: size versus belongingness 42 4.2 Experiment 6: Lightness and perceived size 48 4.3. General discussion 54 Chapter V. How are size and lightness bound in Delboeuf-like displays? 57 5.1 Experiment 7 57 5.2 General discussion 64 Chapter VI. Lightness effects in Ebbinghaus displays 67 6.1 Experiment 8: lightness and the Ebbinghaus illusion 67 6.2 Experiment 9: is the size illusion still there? 70 6.3 General discussion 71 Chapter VII. Conclusions and future research 73 7.1 The point 75 7.2 Future research 76 References 81 Abstract 89 Riassunto 93 II Preface ..the moment at which man lives most fully is when he is seeking something.. -

Intelligence of Bearded Dragons Sydney Herndon
Murray State's Digital Commons Honors College Theses Honors College Spring 4-26-2021 Intelligence of Bearded Dragons sydney herndon Follow this and additional works at: https://digitalcommons.murraystate.edu/honorstheses Part of the Behavior and Behavior Mechanisms Commons Recommended Citation herndon, sydney, "Intelligence of Bearded Dragons" (2021). Honors College Theses. 67. https://digitalcommons.murraystate.edu/honorstheses/67 This Thesis is brought to you for free and open access by the Honors College at Murray State's Digital Commons. It has been accepted for inclusion in Honors College Theses by an authorized administrator of Murray State's Digital Commons. For more information, please contact [email protected]. Intelligence of Bearded Dragons Submitted in partial fulfillment of the requirements for the Murray State University Honors Diploma Sydney Herndon 04/2021 i Abstract The purpose of this thesis is to study and explain the intelligence of bearded dragons. Bearded dragons (Pogona spp.) are a species of reptile that have been popular in recent years as pets. Until recently, not much was known about their intelligence levels due to lack of appropriate research and studies on the species. Scientists have been studying the physical and social characteristics of bearded dragons to determine if they possess a higher intelligence than previously thought. One adaptation that makes bearded dragons unique is how they respond to heat. Bearded dragons optimize their metabolic functions through a narrow range of body temperatures that are maintained through thermoregulation. Many of their behaviors are temperature dependent, such as their speed when moving and their food response. When they are cold, these behaviors decrease due to their lower body temperature. -

A Neuro-Mathematical Model for Size and Context Related Illusions
A neuro-mathematical model for size and context related illusions. B. Franceschiello, A. Sarti, G. Citti March 2019 Abstract We provide here a mathematical model of size/context illusions, inspired by the functional architecture of the visual cortex. We first recall previous models of scale and orientation, in particular [46], and simplify it, only considering the feature of scale. Then we recall the deformation model of illusion, introduced by [16] to describe orientation related GOIs, and adapt it to size illusion. We finally apply the model to the Ebbinghaus and Delboeuf illusions, validating the results by comparing with experimental data from [34] and [44]. 1 Introduction Geometrical-optical illusions (GOIs) are a class of phenomena first discovered by German physicists and physiologists in the late XIX century, among them Oppel and Hering ([39], [22]), and can be defined as situations where a perceptual mismatch between the visual stimulus and its geometrical properties arise [53]. Those illusions are typically analyzed according to the main geometrical features of the stimulus, whether it is contours orientation, contrast, context influence, size or a combination of the above mentioned ones ([53, 38, 11]). arXiv:1908.10162v1 [q-bio.NC] 27 Aug 2019 Figure 1: The Ebbinghaus illusion (left) and the Delboeuf illusion (right) In this work we are mainly interested in size and context related phenomena, a class of stimuli where the size of the surroundings elements induces a misperception of the central target width. In figure 1, two famous effects are presented, the Ebbinghaus and Delboeuf illusions: the presence of circular inducers (figure 1, left) and of an annulus (figure 1, right) varies the perceived sizes of the central targets. -

Vision Biological Vision and Image Processing
Vision Stefano Ferrari Universit`adegli Studi di Milano [email protected] Methods for Image processing academic year 2016–2017 Biological vision and image processing I The human visual perception results from a chain of processing tasks, often cooperating with other perceptual activities. I Image processing is a discipline (mainly) based on mathematical and statistical concepts, with constraints imposed by the image acquisition technology. I The study of the human vision is useful for more than one reason: I the output of some processes is targeted for the vision of a human user; I the operation of the organs devoted to the acquisition and perception of images can inspire the development of similar artificial devices; moreover, the performance of image processing algorithms have to be compared; I some known mulfunctioning of the biological apparatus do not affect the image processing devices. Stefano Ferrari| Methods for Image processing| a.a. 2016/17 1 . Eye I cornea I sclera I pupil I iris I lens I choroid I retina I fovea I blind spot Blind spot: close the left eye, sta- re to the cross, come closer to the screen till the spot disappears. Distribution of the receptors on the retina On the retina, two kinds of receptors can be found: I cones; I rods. I Cones: I about 7 millions; I perception of details and rapid changes; I sensitive to colors; I sensitive in high illuminance conditions (photopic vision). I Rods: I about 100 millions; I provide a large scale vision; I sensitive in low illuminance conditions (scotopic vision). Stefano Ferrari| Methods for Image processing| a.a. -

Sensation and Perception
Sensation and Perception OUTLINE OF RESOURCES Introducing Sensation and Perception Podcast/Lecture/Discussion Topic: Person Perception (p. 3) UPDATED Basic Principles of Sensation and Perception Lecture/Discussion Topics: Sensation Versus Perception (p. 4) Top-Down Processing (p. 5) “Thin-Slicing” (p. 6) Classroom Exercises: A Scale to Assess Sensory-Processing Sensitivity (p. 6) UPDATED Human Senses Demonstration Kits (p. 6) UPDATED Classroom Exercises/Student Projects: The Wundt-Jastrow Illusion (p. 4) LaunchPad Video: The Man Who Cannot Recognize Faces* Thresholds Lecture/Discussion Topics: Gustav Fechner and Psychophysics (p. 7) Subliminal Smells (p. 8) Subliminal Persuasion (p. 9) Applying Weber’s Law (p. 10) Student Projects: The Variability of the Absolute Threshold (p. 8) Understanding Weber’s Law (p. 9) Sensory Adaptation Student Project: Sensory Adaptation (p. 10) UPDATED Classroom Exercises: Eye Movements (p. 10) Sensory Adaptation in the Marketplace (p. 11) Perceptual Set Lecture/Discussion Topic: Do Red Objects Feel Warmer or Colder Than Blue Objects? (p. 11) NEW Classroom Exercises: Perceptual Set (p. 11) UPDATED Perceptual Set and Gender Stereotypes (p. 12) Context Effects (see also Brightness Contrast, p. 22) Lecture/Discussion Topic: Context and Perception (p. 13) Vision: Sensory and Perceptual Processing Classroom Exercise/Student Project: Physiology of the Eye—A CD-ROM for Teaching Sensation and Perception (p. 13) LaunchPad Video: Vision: How We See* The Eye and the Stimulus Input Lecture/Discussion Topic:Classroom as Eyeball (p. 13) Student Projects: Color the Eyeball (p. 13) NEW Locating the Retinal Blood Vessels (p. 13) Student Projects/Classroom Exercises: Rods, Cones, and Color Vision (p. 14) UPDATED Locating the Blind Spot (p. -

Visual Perception Glossary
Visual Perception Glossary Amodal perception The part of an object that is not visible because occlu- ded can be amodally perceived. Amodal perception is different and one step removed from modal percepti- on of real or illusory contours. Apparent motion When an object is presented at two different locati- ons after a brief time interval observers perceive mo- tion. The fi rst empirical investigation was carried out by Sigmund Exner . His aim, as well as the subsequent work by Max Wertheimer , was in establishing that motion was a basic sensation. Attention Refers to the selective processing of some aspect of information, while ignoring other information. The sudden onset of a stimulus can capture attention, but people can also exert some control on where they di- rect their attention. Bi-stable stimulus A particular stimulus that can produce two percepts over time, even though it is unchanged as a stimulus. An example is the Necker cube . Brightness Brightness refers to perception of how much light is coming from a given surface or object. A brighter objects refl ects more light than a less bright object. However perception of brightness is not fully deter- mined by luminance (see Illusory contours). 192 Visual Perception Glossary Cerebral lobe The cerebral cortex of the human brain is divided into four main lobes. Frontal (at the front), occipital (at the back), temporal (on the sides) and parietal (at the top). Consciousness Sorry this is too hard, your guess is as good as mine. Cortex The cortex is the outer layer of the brain . In most mammals the cortex is folded and this allows the surface to have a greater area given in the confi ned space available inside the skull. -

The Blind Spot
Sight (Vision) The Blind Spot One of the most dramatic experiments to perform is the demonstration of the blind spot. The blind spot is the area on the retina without receptors that respond to light. Therefore an image that falls on this region will NOT be seen. It is in this region that the optic nerve exits the eye on its way to the brain. To find your blind spot, look at the image below or draw it on a piece of paper: To draw the blind spot tester on a piece of paper, make a small dot on the left side separated by about 6-8 inches from a small + on the right side. Close your right eye. Hold the image (or place your head from the computer monitor) about 20 inches away. With your left eye, look at the +. Slowly bring the image (or move your head) closer while looking at the +. At a certain distance, the dot will disappear from sight...this is when the dot falls on the blind spot of your retina. Reverse the process. Close your left eye and look at the dot with your right eye. Move the image slowly closer to you and the + should disappear. Here are some more images that will help you find your blind spot. For this image, close your right eye. With your left eye, look at the red circle. Slowly move your head closer to the image. At a certain distance, the blue line will not look broken!! This is because your brain is "filling in" the missing information. -

EBBINGHAUS ILLUSION in TOUCH AS EVIDENCE for the TWO STREAM PERCEPTION-ACTION HYPOTHESIS Erin R
Northern Michigan University NMU Commons All NMU Master's Theses Student Works 8-2014 EBBINGHAUS ILLUSION IN TOUCH AS EVIDENCE FOR THE TWO STREAM PERCEPTION-ACTION HYPOTHESIS Erin R. Smith Northern Michigan University, [email protected] Follow this and additional works at: https://commons.nmu.edu/theses Part of the Psychology Commons Recommended Citation Smith, Erin R., "EBBINGHAUS ILLUSION IN TOUCH AS EVIDENCE FOR THE TWO STREAM PERCEPTION-ACTION HYPOTHESIS" (2014). All NMU Master's Theses. 31. https://commons.nmu.edu/theses/31 This Open Access is brought to you for free and open access by the Student Works at NMU Commons. It has been accepted for inclusion in All NMU Master's Theses by an authorized administrator of NMU Commons. For more information, please contact [email protected],[email protected]. EBBINGHAUS ILLUSION IN TOUCH AS EVIDENCE FOR THE TWO STREAM PERCEPTION-ACTION HYPOTHESIS By Erin Smith THESIS Submitted To Northern Michigan University In partial fulfillment of the Requirements For the degree Of MASTER OF SCIENCE Office of Graduate Education and Research 2014 SIGNATURE APPROVAL FORM Title of Thesis: Ebbinghaus Illusion in Touch as Evidence for the Two-Stream Perception-Action Hypothesis This thesis by Erin R. Smith is recommended for approval by the student’s Thesis Committee and Department Head in the Department of Psychology and by the Assistant Provost of Graduate Education and Research. ____________________________________________________________ Committee Chair: Date ____________________________________________________________ -

Perception ECVP 2010 Abstract Supplement
gover imgeX ferggeist9 y ndro helEreteY opyright ndro helErete @wwwFsndrodelpreteFomA Perception, 2010, volume 39, supplement, page 1 – 203 Thirty-third European Conference on Visual Perception Lausanne, Switzerland 22 – 26 August 2010 Abstracts Sunday Posters: Face perception I 89 Symposium in honour of the late 1 Posters: Motion I 94 Richard Gregory Posters: Natural images 99 Perception Lecture (C Koch) 3 Posters: Objects and scenes 101 Monday Wednesday Talks: Attention 4 Talks: 3D Vision, depth 107 Talks: Adaptation, Brightness 6 Symposium: The complexity of visual 110 and Contrast stability Symposium: Face perception in man 9 Symposium: Psychophysics: 112 and monkey yesterday, today, and tomorrow Talks: Objects 10 Posters: Art and vision 113 Symposium: Colour appearance 12 Posters: Attention II 117 mechanisms Posters: Consciousness and rivalry 123 Talks: Neural mechanisms 14 Posters: Face perception II 125 Posters: Applied vision 16 Posters: Haptics 130 Posters: Biological motion 18 Posters: Imaging 133 Posters: Clinical vision and ageing 20 Posters: Motion II 137 Posters: Contours and crowding 25 Posters: Multisensory processing 142 Posters: Emotions and cognition 30 Posters: Reading 147 Posters: Eye movements I 33 Posters: Learning and memory 38 Thursday Posters: Models and theory 44 Talks: Motion 150 Posters: Non-human vision 49 Talks: Multistability, rivalry 152 Posters: Perception and action I 50 Talks: Face perception 155 Talks: Colour 157 Tuesday Posters: 3D Vision, depth II 160 Talks: Eye movements 55 Posters: Brightness, lightness 165 Talks: Clinical vision 57 Posters: Contrast 168 Talks: Spatial vision 60 Posters: Eye movements II 170 Talks: Learning and memory 62 Posters: Illusions 174 Symposium: Visual social perception: 64 Posters: Perception and action II 180 Brain imaging and sex differences Posters: Perceptual organization 184 Talks: Multisensory processing 66 Posters: Temporal processing 187 and haptics Posters: Visual search 191 Rank Lecture 68 Publisher’s note. -

Undergraduate Honors Projects – 2015-2016
Undergraduate Honors Projects – 2015-2016 David Adams An Underadditivity of the Cellular Mechanisms Responsible for the Orientation Contrast Effects of the Rod-and- Frame Illusion Advisors: Paul Dassonville, Ph.D. and Cris Niell, Ph.D. If a vertical line is surrounded by a tilted frame, it is typically perceived as being tilted in the opposite direction. This rod-and-frame illusion is thought to be driven by two distinct mechanisms. Large frames cause a distortion of the egocentric reference frame, with perceived vertical biased in the direction of the frame’s tilt (i.e., a visuovestibular effect). Small frames are thought to drive the illusion through local contrast effects within early visual processing. Wenderoth and Beh (1977) found that the visuovestibular effect could be induced by a stimulus consisting of only two lines, indicating that an intact frame was not necessary to achieve the illusion. Furthermore, Li and Matin (2005) demonstrated that the Gestalt of an intact frame provided no additional impact to the illusion, as the visuovestibular effect of an intact frame was less than the sum of its parts. It is unclear whether the same is true for the local contrast effects caused by small frames. Participants performed a perceptual task in which they reported the orientation of a target line (12’ in length) presented in the context of either an intact frame (32’ on a side, tilted ± 15°) or partial frame (that is, flankers consisting of either the top and bottom of the frame in collinear locations with respect to the target line, or the left and right sides in lateral locations). -
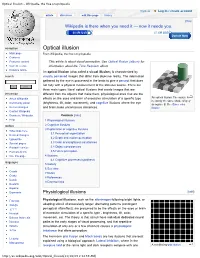
Optical Illusion - Wikipedia, the Free Encyclopedia
Optical illusion - Wikipedia, the free encyclopedia Try Beta Log in / create account article discussion edit this page history [Hide] Wikipedia is there when you need it — now it needs you. $0.6M USD $7.5M USD Donate Now navigation Optical illusion Main page From Wikipedia, the free encyclopedia Contents Featured content This article is about visual perception. See Optical Illusion (album) for Current events information about the Time Requiem album. Random article An optical illusion (also called a visual illusion) is characterized by search visually perceived images that differ from objective reality. The information gathered by the eye is processed in the brain to give a percept that does not tally with a physical measurement of the stimulus source. There are three main types: literal optical illusions that create images that are interaction different from the objects that make them, physiological ones that are the An optical illusion. The square A About Wikipedia effects on the eyes and brain of excessive stimulation of a specific type is exactly the same shade of grey Community portal (brightness, tilt, color, movement), and cognitive illusions where the eye as square B. See Same color Recent changes and brain make unconscious inferences. illusion Contact Wikipedia Donate to Wikipedia Contents [hide] Help 1 Physiological illusions toolbox 2 Cognitive illusions 3 Explanation of cognitive illusions What links here 3.1 Perceptual organization Related changes 3.2 Depth and motion perception Upload file Special pages 3.3 Color and brightness -

The Dynamic Ebbinghaus: Motion Dynamics Greatly Enhance the Classic Contextual Size Illusion
ORIGINAL RESEARCH ARTICLE published: 18 February 2015 HUMAN NEUROSCIENCE doi: 10.3389/fnhum.2015.00077 The Dynamic Ebbinghaus: motion dynamics greatly enhance the classic contextual size illusion Ryan E. B. Mruczek*, Christopher D. Blair , Lars Strother and Gideon P.Caplovitz Department of Psychology, University of Nevada Reno, Reno, NV, USA Edited by: The Ebbinghaus illusion is a classic example of the influence of a contextual surround on Baingio Pinna, University of Sassari, the perceived size of an object. Here, we introduce a novel variant of this illusion called the Italy Dynamic Ebbinghaus illusion in which the size and eccentricity of the surrounding inducers Reviewed by: modulates dynamically over time. Under these conditions, the size of the central circle is Daniele Zavagno, University of Milano-Bicocca, Italy perceived to change in opposition with the size of the inducers. Interestingly, this illusory Dejan Todorovic, University of effect is relatively weak when participants are fixating a stationary central target, less Belgrade, Serbia than half the magnitude of the classic static illusion. However, when the entire stimulus *Correspondence: translates in space requiring a smooth pursuit eye movement to track the target, the Ryan E. B. Mruczek, Department of illusory effect is greatly enhanced, almost twice the magnitude of the classic static illusion. Psychology, University of Nevada, 1664 North Virginia Street, Reno, NV A variety of manipulations including target motion, peripheral viewing, and smooth pursuit 89557-0296, USA eye movements all lead to dramatic illusory effects, with the largest effect nearly four e-mail: [email protected] times the strength of the classic static illusion.