United States Patent Office Patented Mar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

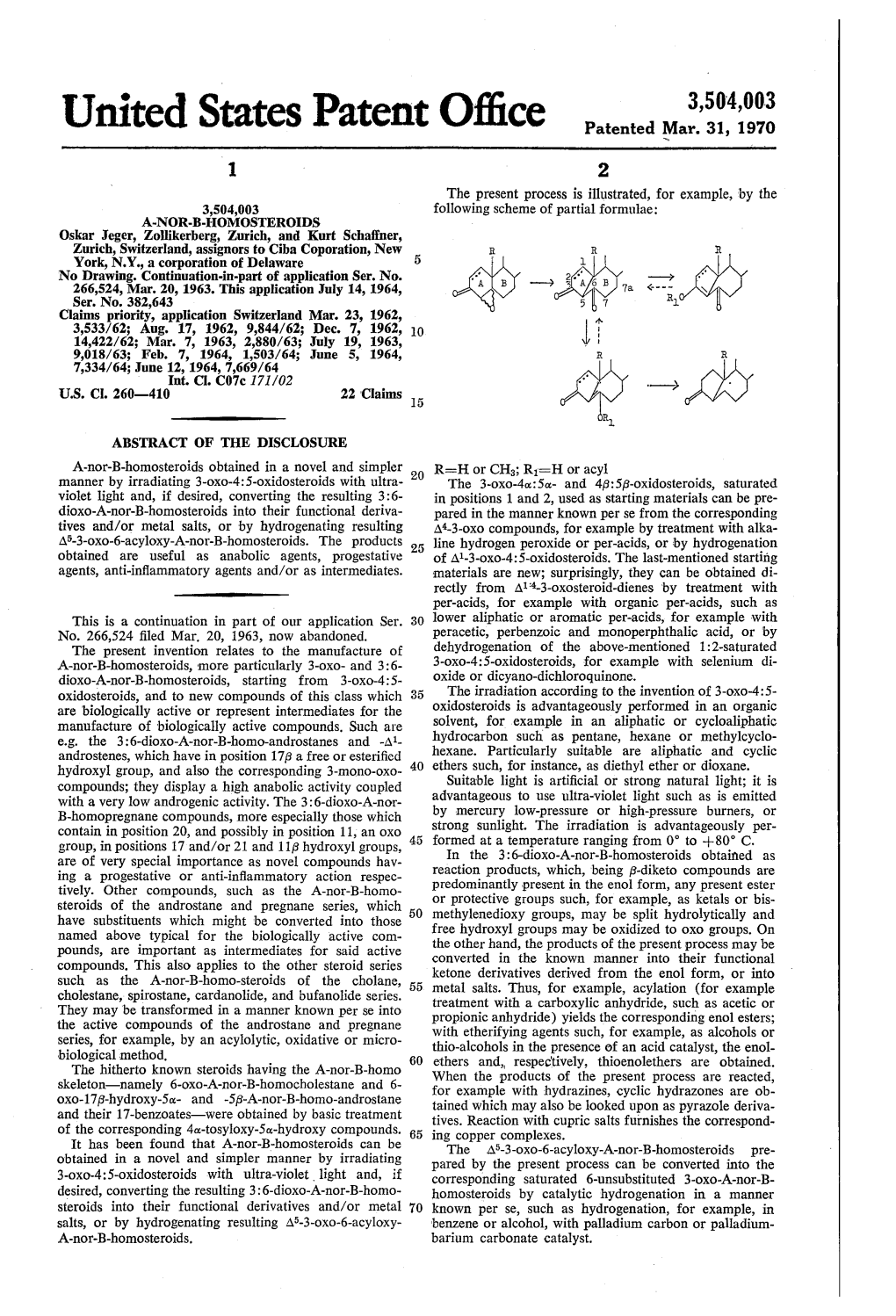
Seco-Steroids C07C)
CPC - C07J - 2021.08 C07J STEROIDS (seco-steroids C07C) Definition statement This place covers: Compounds containing a cyclopenta[a]hydrophenanthrene skeleton (see below) or a ring structure derived therefrom: • by contraction or expansion of one ring by one or two atoms; • by contraction or expansion of two rings each by one atom; • by contraction of one ring by one atom and expansion of one ring by one atom; • by substitution of one or two carbon atoms of the cyclopenta[a]hydrophenanthrene skeleton, which are not shared by rings, by hetero atoms, in combination with the above defined contraction or expansion or not, or; • by condensation with carbocyclic or heterocyclic rings in combination with one or more of the foregoing alterations or not. Preparation of steroids including purification, separation, stabilisation or use of additives unless provided for elsewhere, as specified below. Treatment and modification of steroids provided that • the treatment is not provided for elsewhere and • the resultant product is a compound under the subclass definition. Relationships with other classification places In class C07, in the absence of an indication to the contrary, a compound is classified in the last appropriate place, i.e. in the last appropriate subclass. For example cyclopenta [a] hydrophenantrenes are classified in subclass C07J as steroids and not in subclasses C07C or C07D as carbocyclic or heterocyclic compounds. Subclass C07J is a function-oriented entry for the compounds themselves and does not cover the application or use of the compounds under the subclass definition. For classifying such information other entries in the IPC exist, for example: Subclass A01N: Preservation of bodies of humans or animals or plants or parts thereof; biocides, e.g. -

United States Patent Office Patented Oct
3,211,760 United States Patent Office Patented Oct. 12, 1965 2 Furthermore there may be mentioned: 3,211,760 PROCESS FOR THE MANUFACTURE OF A-3:20-dioxo-10-acetoxy-19-norpregnene, 19-NOR-STERODS A-3:20-dioxo-10:17a-diacetoxy-19-norpregnene, Oskar Jeger and Kurt Schaffner, Zurich, Switzerland, A-3-oxo-10-acetoxy-19-norspirostene, and the 6-dehydro assignors to Ciba Corporation, New York, N.Y., a cor derivatives thereof, such as poration of Delaware A-3: 17-dioxo-103-acetoxy-19-norandrostadiene or No Drawing. Filed May 7, 1963, Ser. No. 278,775 A-3:20-dioxo-103:17a-diacetoxy-19-norpregnadiene. Claims priority, application Switzerland, May 11, 1962, 5,736/62 For the reduction according to the invention there are 19 Claims, (C. 260-397.3) O particularly suitable metallic reducing agents, more es pecially those which are capable of removing the 10-sub The present invention provides a new process for the stituent by reduction and at the same time converting the manufacture of 10-unsubstituted 19-norsteroids from A-3-oxo grouping into a A35(10)-enolate; that is to say steriods that contain an esterified hydroxyl group in posi that there are primarily suitable metals of groups I and tion 10. 15 II of the Periodic System, if desired in combination with 19-norsteroids, more especially derivatives of 19-nortes hydrogen donors, for example, alkali or alkaline earth tosterone and of 19-norprogesterone, have become very metals such as sodium, potassium, lithium or calcium, important in recent years as anabolic and gestagenic medic preferably dissolved in liquid ammonia or in an amine aments and as ovulation inhibitors. -

Nomenclature of Steroids
Pure&App/. Chern.,Vol. 61, No. 10, pp. 1783-1822,1989. Printed in Great Britain. @ 1989 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY and INTERNATIONAL UNION OF BIOCHEMISTRY JOINT COMMISSION ON BIOCHEMICAL NOMENCLATURE* NOMENCLATURE OF STEROIDS (Recommendations 1989) Prepared for publication by G. P. MOSS Queen Mary College, Mile End Road, London El 4NS, UK *Membership of the Commission (JCBN) during 1987-89 is as follows: Chairman: J. F. G. Vliegenthart (Netherlands); Secretary: A. Cornish-Bowden (UK); Members: J. R. Bull (RSA); M. A. Chester (Sweden); C. LiCbecq (Belgium, representing the IUB Committee of Editors of Biochemical Journals); J. Reedijk (Netherlands); P. Venetianer (Hungary); Associate Members: G. P. Moss (UK); J. C. Rigg (Netherlands). Additional contributors to the formulation of these recommendations: Nomenclature Committee of ZUB(NC-ZUB) (those additional to JCBN): H. Bielka (GDR); C. R. Cantor (USA); H. B. F. Dixon (UK); P. Karlson (FRG); K. L. Loening (USA); W. Saenger (FRG); N. Sharon (Israel); E. J. van Lenten (USA); S. F. Velick (USA); E. C. Webb (Australia). Membership of Expert Panel: P. Karlson (FRG, Convener); J. R. Bull (RSA); K. Engel (FRG); J. Fried (USA); H. W. Kircher (USA); K. L. Loening (USA); G. P. Moss (UK); G. Popjiik (USA); M. R. Uskokovic (USA). Correspondence on these recommendations should be addressed to Dr. G. P. Moss at the above address or to any member of the Commission. Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01989 IUPAC), is printed. -

(12) United States Patent (10) Patent No.: US 8,486,374 B2 Tamarkin Et Al
USOO8486374B2 (12) United States Patent (10) Patent No.: US 8,486,374 B2 Tamarkin et al. (45) Date of Patent: Jul. 16, 2013 (54) HYDROPHILIC, NON-AQUEOUS (56) References Cited PHARMACEUTICAL CARRIERS AND COMPOSITIONS AND USES U.S. PATENT DOCUMENTS 1,159,250 A 11/1915 Moulton 1,666,684 A 4, 1928 Carstens (75) Inventors: Dov Tamarkin, Maccabim (IL); Meir 1924,972 A 8, 1933 Beckert Eini, Ness Ziona (IL); Doron Friedman, 2,085,733. A T. 1937 Bird Karmei Yosef (IL); Alex Besonov, 2,390,921 A 12, 1945 Clark Rehovot (IL); David Schuz. Moshav 2,524,590 A 10, 1950 Boe Gimzu (IL); Tal Berman, Rishon 2,586.287 A 2/1952 Apperson 2,617,754 A 1 1/1952 Neely LeZiyyon (IL); Jorge Danziger, Rishom 2,767,712 A 10, 1956 Waterman LeZion (IL); Rita Keynan, Rehovot (IL); 2.968,628 A 1/1961 Reed Ella Zlatkis, Rehovot (IL) 3,004,894 A 10/1961 Johnson et al. 3,062,715 A 11/1962 Reese et al. 3,067,784. A 12/1962 Gorman (73) Assignee: Foamix Ltd., Rehovot (IL) 3,092.255. A 6, 1963 Hohman 3,092,555 A 6, 1963 Horn 3,141,821 A 7, 1964 Compeau (*) Notice: Subject to any disclaimer, the term of this 3,142,420 A 7/1964 Gawthrop patent is extended or adjusted under 35 3,144,386 A 8/1964 Brightenback U.S.C. 154(b) by 1180 days. 3,149,543 A 9, 1964 Naab 3,154,075 A 10, 1964 Weckesser 3,178,352 A 4, 1965 Erickson (21) Appl. -

Downloaded for Personal Non-Commercial Research Or Study, Without Prior Permission Or Charge
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] À Thesis entitled PARTIAL SYNTHESIS AND REGIOSELECTIVE REDUCTION OF STEROIDAL 11,12-SEra-DIOIC ANHYDRIDES Submitted in part fulfilment of the requirements for admittance to the Degree of Doctor of Philosophy by HASSAN ABBAS HASSAN. B.Sc.. M.Sc.. January 1987 Department of Chemistry UNIVERSITY OF OLASOOV ProQuest Number: 10991907 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10991907 Published by ProQuest LLO (2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLO. -

Introduction (Pdf)
Dictionary of Natural Products on CD-ROM This introduction screen gives access to (a) a general introduction to the scope and content of DNP on CD-ROM, followed by (b) an extensive review of the different types of natural product and the way in which they are organised and categorised in DNP. You may access the section of your choice by clicking on the appropriate line below, or you may scroll through the text forwards or backwards from any point. Introduction to the DNP database page 3 Data presentation and organisation 3 Derivatives and variants 3 Chemical names and synonyms 4 CAS Registry Numbers 6 Diagrams 7 Stereochemical conventions 7 Molecular formula and molecular weight 8 Source 9 Importance/use 9 Type of Compound 9 Physical Data 9 Hazard and toxicity information 10 Bibliographic References 11 Journal abbreviations 12 Entry under review 12 Description of Natural Product Structures 13 Aliphatic natural products 15 Semiochemicals 15 Lipids 22 Polyketides 29 Carbohydrates 35 Oxygen heterocycles 44 Simple aromatic natural products 45 Benzofuranoids 48 Benzopyranoids 49 1 Flavonoids page 51 Tannins 60 Lignans 64 Polycyclic aromatic natural products 68 Terpenoids 72 Monoterpenoids 73 Sesquiterpenoids 77 Diterpenoids 101 Sesterterpenoids 118 Triterpenoids 121 Tetraterpenoids 131 Miscellaneous terpenoids 133 Meroterpenoids 133 Steroids 135 The sterols 140 Aminoacids and peptides 148 Aminoacids 148 Peptides 150 β-Lactams 151 Glycopeptides 153 Alkaloids 154 Alkaloids derived from ornithine 154 Alkaloids derived from lysine 156 Alkaloids -
![Systemic Nomenclature of Steroids [Cyclopentaphenanthrene Ring] Their Derivatives](https://docslib.b-cdn.net/cover/9638/systemic-nomenclature-of-steroids-cyclopentaphenanthrene-ring-their-derivatives-5459638.webp)
Systemic Nomenclature of Steroids [Cyclopentaphenanthrene Ring] Their Derivatives
International Journal of Chemical Studies 2016; 4(3): 124-132 P-ISSN2349–8528 E-ISSN 2321–4902 Systemic nomenclature of Steroids IJCS 2016; 4(3): 124-132 © 2016 JEZS [Cyclopentaphenanthrene ring] their derivatives - Received: 18-03-2016 Accepted: 19-04-2016 An Overview Davinder Kumar Davinder Kumar, Virender Kumar and Pawan Jalwal College of Pharmacy University of Health Sciences, Rohtak, India. Abstract More than 25 million chemical compounds currently registered whole over world, about 20-25% contain Virender Kumar heterocyclic systems and Cyclopenta [a] phenanthrene ring containing compounds. Cyclopenta College of Pharmacy University phenanthrene rings (Steroids) are important, not only because of their abundance, but above all because of Health Sciences, Rohtak, of their chemical, biological and technical significance. The numbering, nomenclature and India. stereochemistry of Cyclopenta [a] phenanthrene ring effect their importance and count among their number many natural products, such as vitamins, hormones, antibiotics, alkaloids, as well as Pawan Jalwal Pharmaceuticals aids. Department of Pharmaceutical Sciences, Baba Mastnath Keywords: Steroids, Hydrocarbon, Heterocyclic, Hormone, Nomenclature University, Asthal Bohar, Rohtak India. 1. Introduction Many naturally occurring Cyclopenta [a] phenanthrene (steroidal skeleton) are referred to by their common or trivial names, such as cholesterol, cortisol, progesterone, testosterone, and estradiol etc. As more steroid molecules were being discovered or synthesized, it became clear -

(12) Patent Application Publication (10) Pub. No.: US 2006/0018937 A1 Friedman Et Al
US 20060018937A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0018937 A1 Friedman et al. (43) Pub. Date: Jan. 26, 2006 (54) STEROID KIT AND FOAMABLE Oct. 25, 2002 (IL)................................................. 1524.86 COMPOSITION AND USES THEREOF (75) Inventors: Doron Friedman, Karmei Yosef (IL); Publication Classification Alex Besonov, Rehovet (IL); Dov Tamarkin, Maccabim (IL); Meir Eini, (51) Int. Cl. Ness Ziona (IL) A6IK 8/02 (2006.01) Correspondence Address: (52) U.S. Cl. .............................................................. 424/401 WILMER CUTLER PICKERING HALE AND DORR LLP (57) ABSTRACT 60 STATE STREET BOSTON, MA 02109 (US) A composition and therapeutic kit including an aeroSol packaging assembly including a container accommodating a (73) Assignee: Foamix Ltd. preSSurized product and an outlet capable of releasing a foamable composition, including a Steroid as a foam. The (21) Appl. No.: 11/114,410 preSSurized product includes a foamable composition including: a container accommodating a pressurized prod (22) Filed: Apr. 26, 2005 uct; and an outlet capable of releasing the pressurized Related U.S. Application Data product as a foam; wherein the pressurized product com O prises a foamable composition including: i. a steroid; ii. at (63) Continuation-in-part of application No. 10/911,367, least one organic carrier selected from the group consisting filed on Aug. 4, 2004. of hydrophobic Organic carrier, a polar solvent, an emol Continuation-in-part of application No. 10/532,618, lient and mixtures thereof, at a concentration of about 2% to filed as 371 of international application No. PCT/ about 50% by weight; iii. a surface-active agent, IV. -

Registry File Basic Name Segment Dictionary
Registry File Basic Name Segment Dictionary June 1993 STN International 2540 Olentangy River 'Road P.O. Box 02228 Columbus, OH 43202 Copyright Q 1993 American Chemical Society Quoting or copying of material from this publication for educational purposes is encouraged, providing acknowledgement is made of the source of such material. The Basic lndex of the REGISTRY File contains name fragments, molecular formula fragments, and Collective lndex (CI) codes. The names of the substances have been segmented (parsed) to provide access to nomenclature terms that may be embedded within a longer name. Names have been segmented at 3 levels for the Basic Index: natural segments, basic (smallest) segments, and recom- bined basic segments. This document lists the segment dictionary that is used to identify the basic segments for names in the REGISTRY Dictionary File. The segments in this dictionary are nomenclature terms that indicate a chemical functionality or moiety, multiplicative prefix, ring name, or common natural product name. This dictionary can be used to help you to select appropriate basic segments for effective searches in the Basic Index. To do this, it helps to understand how the system evaluates names to produce the basic segments. Since the basic segments are generated from the natural segments, natural seg- ments are briefly discussed first. Natural Segments To create the natural segments, the parentheses andlor brackets in ring designations, e.g., [def,mno] or [2.2.1], and locants, e.g., (1,I), are removed. The resulting character strings are posted to the Basic lndex without further segmentation. Then, all punctuation, except for apostrophes ('), is removed from the remainder of the name. -

Definitive Rules for Nomenclature of Steroids
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY AND INTERNATIONAL UNION OF BIOCHEMISTRY DEFINITIVE RULES FOR NOMENCLATURE OF STEROIDS Issued by the IUPAC Commission on the Nomenclature of Organic Chemistry and IUPA ('—JUB Commission on Biochemical Nomenclature 1971 LONDON BUTTER WORTHS P.A.C.—31----I/2-—M DEFINITIVE RULES FOR NOMENCLATURE OF STEROIDSt CONTENTS Page Introduction 285 2S—1 General 287 25—2 Fundamental carbocycles 291 2S—3 Pentacyelic and hexacyclic modifications 296 2S—4 Derivatives 303 2S—5 Stereochemical modifications 306 25—6 Shortening of sidechains and elimination of methyl groups 310 2S—7 Ring contraction or expansion 311 25—8 Ring fission 314 2S—9 Modification by bond migration (abeo system) 314 2S—lO Heterocyclic modifications 316 2S—11 Steroid alkaloids 316 Appendix: Guidelines for steroids containing additional rings 318 INTRODUCTION The rules for steroid nomenclature originate from a discussion held at the Ciba Foundation in London, England, in 1950 between the representatives of many schools. These were published in diem. & md., London, (195!) pp SN 1-41, and also in French and German. They were subsequently taken over by the International Union of Pure and Applied Chemistry and published in an official form in the Comptes Rendus of the ZUrich meeting in 1952 t These Rules shall be known as the IUPAC—IUB 1971 Definitive Rules for Steroid Nomenclature. These Rules are issued by the IUPAC Commission on the Nomenclature of Organic Chemistry and by the IUPAC—IUB Commission on Biochemical Nomenclature. Those who have served on the Commission on the Nomenclature of Organic Chemistry for varying periods during 1967—71 are the following. -

Volume 2 Section C Chemistry; Metallurgy
International Patent Classification Core Level (2010.01) Volume 2 Section C Chemistry; Metallurgy World Intellectual Property Organization SECTION C --- CHEMISTRY; METALLURGY CHEMISTRY C05G Mixtures of fertilisers covered individually by different subclasses of class C05; Mixtures of one or more fertilisers with materials not having a specific fertilising activity, e.g. pesticides, soil- C01 INORGANIC CHEMISTRY ...........................................8 conditioners, wetting agents; Fertilisers characterised by their form............................................... 22 C01B Non-metallic elements; Compounds thereof ......................8 C01C Ammonia; Cyanogen; Compounds thereof ........................9 C01D Compounds of alkali metals, i.e. lithium, sodium, C06 EXPLOSIVES; MATCHES .......................................... 23 potassium, rubidium, caesium, or francium .....................10 C01F Compounds of the metals beryllium, magnesium, C06B Explosive or thermic compositions; Manufacture aluminium, calcium, strontium, barium, radium, thereof; Use of single substances as explosives............... 23 thorium, or of the rare-earth metals ..................................10 C06C Detonating or priming devices; Fuses; Chemical C01G Compounds containing metals not covered by lighters; Pyrophoric compositions.................................... 24 subclasses C01D Or C01F ................................................11 C06D Means for generating smoke or mist; Gas-attack compositions; Generation of gas for blasting or propulsion (chemical -

Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013
International Union of Pure and Applied Chemistry Division VIII Chemical Nomenclature and Structure Representation Division Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4 Chapter P-10 PARENT STRUCTURES FOR NATURAL PRODUCTS AND RELATED COMPOUNDS P-100 Introduction P-101 Nomenclature for natural products based on parent hydrides (alkaloids, steroids, terpenes, carotenes, corrinoids, tetrapyrroles, and similar compounds) P-102 Carbohydrate nomenclature P-103 Amino acids and peptides P-104 Cyclitols P-105 Nucleosides P-106 Nucleotides P-107 Lipids P-100 INTRODUCTION In the field of natural products, three levels of nomenclature are recognized. A new compound, isolated from a natural source, is generally given a ‘trivial’ name. By common usage, these trivial names are commonly related to the biological origin of the material, but frequently not in a rational way, since the available structure is not known with great detail. These trivial names are considered to be ephemeral and replaced for chemical purposes by names describing the skeleton, the characteristic groups, and the organyl substituent groups. When the full structure is known, a ‘systematic name’ may be generated in accordance with Rules described in Chapters P-1 through P-9 of these recommendations. However, this name may be too cumbersome to be continually inserted into the text of a scientific paper. To overcome this difficulty and show the close similarity to related compounds, a ‘semisystematic name’ can be formed. Preferred IUPAC names (PINs) are not identified for the compounds in this Chapter.