Redescription and Molecular Identification of Isospora Feroxis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphological and Molecular Identification of Isospora
Acta Protozool. (2019) 58: 17–23 www.ejournals.eu/Acta-Protozoologica ACTA doi:10.4467/16890027AP.19.007.10838 PROTOZOOLOGICA Morphological and Molecular Identification of Isospora sepetibensis (Chromista: Miozoa: Eimeriidae) from a New Host, Trichothraupis melanops (Passeriformes: Thraupidae: Tachyphoninae) in South America Jhon Lennon GENOVEZ-OLIVEIRA1, Sergian V. CARDOZO2, Águida A. de OLIVEIRA3, Viviane M. de LIMA3, Ildemar FERREIRA3, Bruno P. BERTO3 1 Programa de Pós-Graduação em Biologia Animal, Universidade Federal Rural do Rio de Janeiro (UFRRJ), Brazil 2 Programa de Pós-Graduação em Biomedicina Translacional, Universidade do Grande Rio, Rua Professor José de Souza Herdy 1160, 25071-202 Duque de Caxias, RJ, Brazil 3 Departamento de Biologia Animal, Instituto de Ciências Biológicas e da Saúde, UFRRJ, RJ, Brazil Abstract. Isospora sepetibensis Berto, Flausino, Luz, Ferreira and Lopes, 2008 is a protozoan coccidian parasite (Chromista: Miozoa: Coc- cidiomorphea: Coccidia) that was originally described from Brazilian tanagers Ramphocelus bresilius (Linnaeus, 1766) in the Marambaia Island in the Coast of the State of Rio de Janeiro. In the current work, this species was identified from black-goggled tanagersTrichothraupis melanops (Vieillot, 1818) in the Itatiaia National Park, which is a protected area with a high degree of vulnerability in the interior of the State of Rio de Janeiro, distant in more than 100 km of the type-locality. Its oocysts are sub-spherical to elongate ovoidal, 25.9 × 20.7 µm with smooth, bi-layered wall, ~ 1.3 µm and length/width ratio of 1.1–1.4 (1.26). Micropyle and oocyst residuum absent, but one or two polar granules are present. -

Reference File
References added since publication of 2007 CRC Handbook of Avian Body Masses Abadie, K. B., J. Pérez Z., and M. Valverde. 2006. Primer reporte de colonias del Martín Peruano Progne murphyi. Cotinga 24:99-101. Ackerman, J. T., J. Y. Takekawa, J. D. Bluso, J. L. Yee, and C. A. Eagles-Smith. 2008. Gender identification of Caspian Terns using external morphology and discriminant function analysis. Wilson Journal of Ornithology 120:378-383. Alarcos, S., C. de la Cruz, E. Solís, J. Valencia, and M. J. García-Baquero. 2007. Sex determination of Iberian Azure-winged Magpies Cyanopica cyanus cooki by discriminant analysis of external measurements. Ringing & Migration 23:211-216. Albayrak, T., A. Besnard, and A. Erdoğan. 2011. Morphometric variation and population relationships of Krüeper’s Nuthatch (Sitta krueperi) in Turkey. Wilson Journal of Ornithology 123:734-740. Aleixo, A., C. E. B. Portes, A. Whittaker, J. D. Weckstein, L. Pedreira Gonzaga, K. J. Zimmer, C. C. Ribas, and J. M. Bates. 2013. Molecular systematics and taxonomic revision of the Curve-billed Scythebill complex (Campylorhamphus procurvoides: Dendrocolaptidae), with description of a new species from western Amazonian Brazil. Pp. 253-257, In: del Hoyo, J., A Elliott, J. Sargatal, and D.A. Christie (eds). Handbook of the birds of the world. Special volume: new species and global index. Lynx Edicions, Barcelona, Spain. Volume 1. Alfano, A. 2014. Pygmy Nightjar (Nyctopolus hirundinaeus). Neotropical Birds Online (T.S. Schulenberg, ed.). Cornell Laboratory of Ornithology, Ithaca, NY. Alvarenga, H. M. F., E. Höfling, and L. F. Silveira. 2002. Notharchus swainsoni (Gray, 1846) é uma espécie válida. -

Brazil's Eastern Amazonia
The loud and impressive White Bellbird, one of the many highlights on the Brazil’s Eastern Amazonia 2017 tour (Eduardo Patrial) BRAZIL’S EASTERN AMAZONIA 8/16 – 26 AUGUST 2017 LEADER: EDUARDO PATRIAL This second edition of Brazil’s Eastern Amazonia was absolutely a phenomenal trip with over five hundred species recorded (514). Some adjustments happily facilitated the logistics (internal flights) a bit and we also could explore some areas around Belem this time, providing some extra good birds to our list. Our time at Amazonia National Park was good and we managed to get most of the important targets, despite the quite low bird activity noticed along the trails when we were there. Carajas National Forest on the other hand was very busy and produced an overwhelming cast of fine birds (and a Giant Armadillo!). Caxias in the end came again as good as it gets, and this time with the novelty of visiting a new site, Campo Maior, a place that reminds the lowlands from Pantanal. On this amazing tour we had the chance to enjoy the special avifauna from two important interfluvium in the Brazilian Amazon, the Madeira – Tapajos and Xingu – Tocantins; and also the specialties from a poorly covered corner in the Northeast region at Maranhão and Piauí states. Check out below the highlights from this successful adventure: Horned Screamer, Masked Duck, Chestnut- headed and Buff-browed Chachalacas, White-crested Guan, Bare-faced Curassow, King Vulture, Black-and- white and Ornate Hawk-Eagles, White and White-browed Hawks, Rufous-sided and Russet-crowned Crakes, Dark-winged Trumpeter (ssp. -

(Hymenoptera: Vespidae) and Tolmomyias Sulphurescens Spix
doi:10.12741/ebrasilis.v10i1.638 e-ISSN 1983-0572 Publication of the project Entomologistas do Brasil www.ebras.bio.br Creative Commons Licence v4.0 (BY-NC-SA) Copyright © EntomoBrasilis Copyright © Author(s) Scientific Note/Comunicação Científica Nesting associations between Chartergus globiventris Saussure (Hymenoptera: Vespidae) and Tolmomyias sulphurescens Spix (Passeriformes: Tyrannidae) in southeastern Brazil Marcos Magalhães Souza¹, Ângela Gomes Brunismann¹ & Epifânio Porfiro Pires² 1. Instituto Federal do Sul de Minas, Campus Inconfidentes. 2. Departamento de Entomologia, Universidade Federal de Lavras. EntomoBrasilis 10 (1): 51-53 (2017) Abstract. The success of social wasps is highly dependent on nest construction and colony maintenance. Species use different strategies to avoid nest predation, including forming associations with other insects and vertebrates. This study describes for the first time the association between the social wasp Chartergus globiventris Saussure and the yellow-olive flycatcher Tolmomyias sulphurescens Spix in a deciduous seasonal forest fragment in southeastern Brazil. We located eight active C. globiventris colonies in the study site, three of which were associated with active T. sulphurescens nests. Bird-wasp associations in previous studies have been regarded as commensalism because only birds seem to benefit. However, further studies are needed to better understand the potential benefits of this relationship for both taxa. Keywords: Bird nest; Hymenoptera; interaction; nesting; social wasp. Associações de nidificação entreChartergus globiventris Saussure (Hymenoptera: Vespidae) e Tolmomyias sulphurescens Spix (Passeriformes: Tyrannidae) no sudeste do Brasil Resumo. O sucesso das espécies de vespas sociais está relacionado tanto a construção quanto a manutenção das colônias. Várias espécies utilizam de diversas estratégias para evitar a predação de seus ninhos, como a associação com outros insetos e vertebrados. -

Southeastern Brazil: Best of the Atlantic Forest
SOUTHEASTERN BRAZIL: BEST OF THE ATLANTIC FOREST OCTOBER 8–22, 2017 A trip first, the rarely seen Buff-fronted Owl – Photo: Andrew Whittaker LEADER : ANDREW WHITTAKER LIST COMPILED BY : ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS , INC . 2525 WALLINGWOOD DRIVE , SUITE 1003 AUSTIN , TEXAS 78746 WWW .VENTBIRD .COM SOUTHEASTERN BRAZIL: BEST OF THE ATLANTIC FOREST OCTOBER 8–22, 2017 By Andrew Whittaker Once again, our Brazilian flagship tour visiting the lovely southeast rocked, delivering a bonanza of Atlantic Forest endemics, spectacular scenery, all-around great birding, and wonderful Brazilian cuisine that we have come to expect from this fantastic biologically rich region. First and foremost we tallied 391 species , a whopping 140 of which were regional and/or Brazilian endemics! These figures become all the more impressive when you consider that many of the wider ranging species not included as “endemics” in the preceding tallies are represented in southeast Brazil by distinctive subspecies endemic to the Atlantic Forest region, and that at least 15–25 of these subspecies that we recorded during our tours are likely to be elevated to separate species status in the near future! Beginning in São Paulo, our first destination was Intervales State Park, my own personal favorite among the many great birding spots included in our southeast Brazil trip. Intervales never fails to deliver a huge serving of Atlantic Forest endemics and just plain fantastic birding experiences, and such was the case again this trip. We began the first evening with one of my personal highlights, the fabulous and incredibly cooperative male Long-trained Nightjar . In fact, the male Long-trained Nightjars put on a show for us on two consecutive nights, treating us to multiple close passes, with two males chasing each other just above our heads. -

MORPHOLOGICAL and ECOLOGICAL EVOLUTION in OLD and NEW WORLD FLYCATCHERS a Dissertation Presented to the Faculty of the College O
MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Clay E. Corbin August 2002 This dissertation entitled MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS BY CLAY E. CORBIN has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Associate Professor, Department of Biological Sciences Leslie A. Flemming Dean, College of Arts and Sciences CORBIN, C. E. Ph.D. August 2002. Biological Sciences. Morphological and Ecological Evolution in Old and New World Flycatchers (215pp.) Director of Dissertation: Donald B. Miles In both the Old and New Worlds, independent clades of sit-and-wait insectivorous birds have evolved. These independent radiations provide an excellent opportunity to test for convergent relationships between morphology and ecology at different ecological and phylogenetic levels. First, I test whether there is a significant adaptive relationship between ecology and morphology in North American and Southern African flycatcher communities. Second, using morphological traits and observations on foraging behavior, I test whether ecomorphological relationships are dependent upon locality. Third, using multivariate discrimination and cluster analysis on a morphological data set of five flycatcher clades, I address whether there is broad scale ecomorphological convergence among flycatcher clades and if morphology predicts a course measure of habitat preference. Finally, I test whether there is a common morphological axis of diversification and whether relative age of origin corresponds to the morphological variation exhibited by elaenia and tody-tyrant lineages. -

Describing Species
DESCRIBING SPECIES Practical Taxonomic Procedure for Biologists Judith E. Winston COLUMBIA UNIVERSITY PRESS NEW YORK Columbia University Press Publishers Since 1893 New York Chichester, West Sussex Copyright © 1999 Columbia University Press All rights reserved Library of Congress Cataloging-in-Publication Data © Winston, Judith E. Describing species : practical taxonomic procedure for biologists / Judith E. Winston, p. cm. Includes bibliographical references and index. ISBN 0-231-06824-7 (alk. paper)—0-231-06825-5 (pbk.: alk. paper) 1. Biology—Classification. 2. Species. I. Title. QH83.W57 1999 570'.1'2—dc21 99-14019 Casebound editions of Columbia University Press books are printed on permanent and durable acid-free paper. Printed in the United States of America c 10 98765432 p 10 98765432 The Far Side by Gary Larson "I'm one of those species they describe as 'awkward on land." Gary Larson cartoon celebrates species description, an important and still unfinished aspect of taxonomy. THE FAR SIDE © 1988 FARWORKS, INC. Used by permission. All rights reserved. Universal Press Syndicate DESCRIBING SPECIES For my daughter, Eliza, who has grown up (andput up) with this book Contents List of Illustrations xiii List of Tables xvii Preface xix Part One: Introduction 1 CHAPTER 1. INTRODUCTION 3 Describing the Living World 3 Why Is Species Description Necessary? 4 How New Species Are Described 8 Scope and Organization of This Book 12 The Pleasures of Systematics 14 Sources CHAPTER 2. BIOLOGICAL NOMENCLATURE 19 Humans as Taxonomists 19 Biological Nomenclature 21 Folk Taxonomy 23 Binomial Nomenclature 25 Development of Codes of Nomenclature 26 The Current Codes of Nomenclature 50 Future of the Codes 36 Sources 39 Part Two: Recognizing Species 41 CHAPTER 3. -

Adrenal and Thyroid Weights in Birds
July• 397 1961] ADRENAL AND THYROID WEIGHTS IN BIRDS FRANK A. I•ARTMAN AND t•ATItARINE A. BROWNELL LITTLE studyhas beenmade of the relative sizesof adrenaland thy- roid glandsin different speciesof birds. One of us (Hartman, 1946) reported such a study on birds collectedin the United States some years ago. In this paper, resultson birds collectedin Panamainclude a numberof familiesand speciesnot found in the United States, some migrants from the United States, and two domesticspecies, Gallus gallus and Coturnix coturnix. Data from 249 speciesin 49 familiesare reported. Specimenswere obtained during Decemberand January near sea level on the Rio Chagresand during February and March at 1,300 meters elevation near the villageof E1 VolcAnin the Provinceof Chiriqui. This material was also usedto study the musclesof locomotionas well as to furnish skins of the rare forms. METHODS All specimenswere kept in plastic,waterproof bags to preventdrying until weighed at the field station. Small birds were weighed on a torsionbalance of 120 gramscapacity. Larger oneswere weighedon Chatilion spring balances,the most sensitiveone for the weight in- volved being used: 6,000 grams capacitywith 24 grams sensitivity; 500 gramscapacity with 10 gramssensitivity; and 250 gramscapacity with 5 grams sensitivity. The adrenalsand thyroids were carefully dissectedfree of extraneoustissue with the aid of a binocularloupe and promptlyweighed on a Roller-Smith balanceof either 30 mg. or 1,500 mg. capacity,depending upon the size of the specimen.The thorough- ness of the dissectionis extremely important becausemore or less extraneoustissue may adhereto the gland,especially the adrenal,thus contributingto the error. Only birdsof healthyappearance were used. Most birds were collected between 0700 and 1100. -

New Species Discoveries in the Amazon 2014-15
WORKINGWORKING TOGETHERTOGETHER TO TO SHARE SCIENTIFICSCIENTIFIC DISCOVERIESDISCOVERIES UPDATE AND COMPILATION OF THE LIST UNTOLD TREASURES: NEW SPECIES DISCOVERIES IN THE AMAZON 2014-15 WWF is one of the world’s largest and most experienced independent conservation organisations, WWF Living Amazon Initiative Instituto de Desenvolvimento Sustentável with over five million supporters and a global network active in more than 100 countries. WWF’s Mamirauá (Mamirauá Institute of Leader mission is to stop the degradation of the planet’s natural environment and to build a future Sustainable Development) Sandra Charity in which humans live in harmony with nature, by conserving the world’s biological diversity, General director ensuring that the use of renewable natural resources is sustainable, and promoting the reduction Communication coordinator Helder Lima de Queiroz of pollution and wasteful consumption. Denise Oliveira Administrative director Consultant in communication WWF-Brazil is a Brazilian NGO, part of an international network, and committed to the Joyce de Souza conservation of nature within a Brazilian social and economic context, seeking to strengthen Mariana Gutiérrez the environmental movement and to engage society in nature conservation. In August 2016, the Technical scientific director organization celebrated 20 years of conservation work in the country. WWF Amazon regional coordination João Valsecchi do Amaral Management and development director The Instituto de Desenvolvimento Sustentável Mamirauá (IDSM – Mamirauá Coordinator Isabel Soares de Sousa Institute for Sustainable Development) was established in April 1999. It is a civil society Tarsicio Granizo organization that is supported and supervised by the Ministry of Science, Technology, Innovation, and Communications, and is one of Brazil’s major research centres. -

TOP BIRDING LODGES of PANAMA with the Illinois Ornithological Society
TOP BIRDING LODGES OF PANAMA WITH IOS: JUNE 26 – JULY 5, 2018 TOP BIRDING LODGES OF PANAMA with the Illinois Ornithological Society June 26-July 5, 2018 Guides: Adam Sell and Josh Engel with local guides Check out the trip photo gallery at www.redhillbirding.com/panama2018gallery2 Panama may not be as well-known as Costa Rica as a birding and wildlife destination, but it is every bit as good. With an incredible diversity of birds in a small area, wonderful lodges, and great infrastructure, we tallied more than 300 species while staying at two of the best birding lodges anywhere in Central America. While staying at Canopy Tower, we birded Pipeline Road and other lowland sites in Soberanía National Park and spent a day in the higher elevations of Cerro Azul. We then shifted to Canopy Lodge in the beautiful, cool El Valle de Anton, birding the extensive forests around El Valle and taking a day trip to coastal wetlands and the nearby drier, more open forests in that area. This was the rainy season in Panama, but rain hardly interfered with our birding at all and we generally had nice weather throughout the trip. The birding, of course, was excellent! The lodges themselves offered great birding, with a fruiting Cecropia tree next to the Canopy Tower which treated us to eye-level views of tanagers, toucans, woodpeckers, flycatchers, parrots, and honeycreepers. Canopy Lodge’s feeders had a constant stream of birds, including Gray-cowled Wood-Rail and Dusky-faced Tanager. Other bird highlights included Ocellated and Dull-mantled Antbirds, Pheasant Cuckoo, Common Potoo sitting on an egg(!), King Vulture, Black Hawk-Eagle being harassed by Swallow-tailed Kites, five species of motmots, five species of trogons, five species of manakins, and 21 species of hummingbirds. -
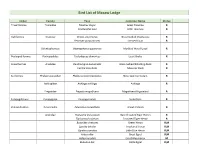
Bird List of Macaw Lodge
Bird List of Macaw Lodge Order Family Taxa Common Name Status Tinamiformes Tnamidae Tinamus major Great Tinamou R Crypturellus soul Little Tinamou R Galliformes Cracidae Ortalis cinereiceps Gray-headed Chachalaca R Penelope purpurascens Crested Guan R Odontophoridae Odontophorus gujanensis Marbled Wood-Quail R Podicipediformes Podicipedidae Tachybaptus dominicus Least Grebe R Anseriformes Anatidae Dendrocygna autumnalis Black-bellied Whistling-Duck R Cairina moschata Muscovy Duck R Suliformes Phalacrocoracidae Phalacrocorax brasilianus Neotropic Cormorant R Anhingidae Anhinga anhinga Anhinga R Fregatidae Fregata magnificens Magnificent Frigatebird R Eurypygiformes Eurypygidae Eurypyga helias Sunbittern R Pelecaniformes Pelecanidae Pelecanus occidentalis Brown Pelican R Ardeidae Tigrisoma mexicanum Bare-throated Tiger-Heron R Tigrisoma fasciatum Fasciated Tiger-Heron R Butorides virescens Green Heron R,M Egretta tricolor Tricolored Heron R,M Egretta caerulea Little Blue Heron R,M Ardea alba Great Egret R,M Ardea herodias Great Blue Heron M Bubulcus ibis Cattle Egret R,M Egretta thula Snowy Egret R,M Nyctanassa violacea Yellow-crowned Night-Heron R,M Cochlearius cochlearius Boat-billed Heron R Threskiornithidae Eudocimus albus White Ibis R Mesembrinibis cayennensis Green Ibis R Platalea ajaja Roseate Sponbill R Charadriiformes Charadriidae Vanellus chilensis Southern Lapwing R Charadrius semipalmatus Semipalmated Plover M Recurvirostridae Himantopus mexicanus Black-necked Stilt R,M Scolopacidae Tringa semipalmata Willet M Tringa solitaria -

Brazil I & II Trip Report
North Eastern Brazil I & II Trip Report 20th October to 15th November 2013 Araripe Manakin by Forrest Rowland Trip report compiled by Tour leaderForrest Rowland NE Brazil I tour Top 10 birds as voted by participants: 1. Lear’s Macaw 2. Araripe Manakin 3. Jandaya Parakeet Trip Report - RBT NE Brazil I & II 2013 2 4. Fringe-backed Fire-eye 5. Great Xenops 6. Seven-coloured Tanager 7. Ash-throated Crake 8. Stripe-backed Antbird 9. Scarlet-throated Tanager 10. Blue-winged Macaw NE Brazil II tour Top 10 as voted by participants: 1. Ochre-rumped Antbird 2. Giant Snipe 3. Ruby-topaz Hummingbird (Ruby Topaz) 4. Hooded Visorbearer 5. Slender Antbird 6. Collared Crescentchest 7. White-collared Foliage-Gleaner 8. White-eared Puffbird 9. Masked Duck 10. Pygmy Nightjar NE Brazil is finally gaining the notoriety it deserves among wildlife enthusiasts, nature lovers, and, of course, birders! The variety of habitats that its vast borders encompass, hosting more than 600 bird species (including over 100 endemics), range from lush coastal rainforests, to xerophytic desert-like scrub in the north, across the vast cerrado full of microhabitats both known as well as entirely unexplored. This incredible diversity, combined with emerging infrastructure, a burgeoning economy paying more attention to eco-tourism, and a vibrant, dynamic culture makes NE Brazil one of the planet’s most unique and rewarding destinations to explore. The various destinations we visited on these tours allowed us time in all of these diverse habitat types – and some in the Lear’s Macaws by Forrest Rowland most spectacular fashion! Our List Totals of 501 birds and 15 species of mammals (including such specials as Lear’s Macaw, Araripe Manakin, Bahia Tapaculo, and Pink-legged Graveteiro) reflect not only how diverse the region is, but just how rich it can be from day to day.