Toxicological Profile for Hexachlorobenzene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
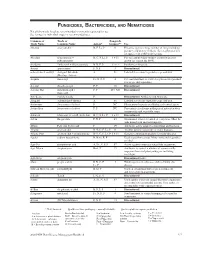
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Concentration, Reduction Efficancy and Degradation of Chlorothalonil and Lambda Cyhalothrin Pesticides in Vegetables Sold in a Nairobi City Market
UNIVERSITY OF NAIROBI CONCENTRATION, REDUCTION EFFICANCY AND DEGRADATION OF CHLOROTHALONIL AND LAMBDA CYHALOTHRIN PESTICIDES IN VEGETABLES SOLD IN A NAIROBI CITY MARKET BY JAMES MUNGAI I56/80825/2015 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR AWARD OF THE DEGREE OF MASTER OF SCIENCE IN ANALYTICAL CHEMISTRY OF THE UNIVERSITY OF NAIROBI AUGUST 2020 i DECLARATION I declare that this research work is my original work and has not been submitted to any institution for any academic award. Where other people’s works have been cited, this has properly been acknowledged and referenced in accordance with the University of Nairobi’s requirements. SIGN……………………………… DATE……………….............. JAMES MUNGAI I56/80825/2015 This thesis is submitted for examination with our approval as the university supervisors. Signature ………………………….. Date …………………… DR. JOYCE G. N. KITHURE DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI Signature ………………………….. Date …………………… DR. VINCENT O. MADADI DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI Signature ………………………….. Date …………………… DR. DEBORAH A. ABONG’O DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI ii DEDICATION This research work is dedicated to my inspiration and mentor the late Professor G.N. Kamau for his selfless support, guidance and professional advice during early stages of this study. iii ACKNOWLEDGEMENT In the course of my research work, I have enjoyed inspiration, love, support, guidance and co-operation. I am very grateful to God for the opportunity He has given to me to undertake this study. I give special thanks posthumously to the late Prof. G. N. Kamau for his inspiration, professional advice and valuable guidance. I am very grateful to Dr. Joyce G. N. -

SDS # : FO001139-1-A Revision Date: 2016-05-26 Format: NA Version 1.02
SAFETY DATA SHEET Fame™ +C SC Fungicide SDS # : FO001139-1-A Revision date: 2016-05-26 Format: NA Version 1.02 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Fame™ +C SC Fungicide Other means of identification Product Code(s) FO001139-1-A Synonyms FLUOXASTROBIN: (E)-{ 2-[6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yloxy]phenyl} (5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime (IUPAC name); (1E)-[2-[[6-(2-chlorophenoxy)-5-fluoro-4-pyrimidinyl]oxy]phenyl](5,6-dihydro-1,4,2-dioxazin- 3-yl)methanone O-methyloxime (CAS name) CHLOROTHALONIL: tetrachloroisophthalonitrile (IUPAC name); 2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile (CAS name) Active Ingredient(s) Fluoxastrobin, Chlorothalonil Chemical Family Strobiluron fungicide, Broad spectrum, nonsystemic fungicide Recommended use of the chemical and restrictions on use Recommended Use: Fungicide Restrictions on Use: Use as recommended by the label Manufacturer Address FMC Corporation 2929 Walnut Street Philadelphia, PA 19104 (215) 299-6000 (General Information) [email protected] (E-Mail General Information) Emergency telephone number For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) Medical Emergencies: 1 800 / 331-3148 (PROSAR - U.S.A. & Canada) 1 651 / 632-6793 (PROSAR - All Other Countries - Collect) 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute -

Chemical Hazards Chemist Edson Haddad 2016 Sao Paulo
ENVIRONMENTAL COMPANY OF SAO PAULO STATE – CETESB REGIONAL CENTRE OF STOCKHOLM CONVENTION ON POPs FOR LATIN AMERICA AND THE CARIBBEAN REGION V INTERNATIONAL TRAINING PROGRAM ON ENVIRONMENTAL SOUND MANAGEMENT ON CHEMICALS AND WASTES, ESPECIALLY ON PERSISTENT ORGANIC POLLUTANTS (POPs) AND MERCURY (Hg) Chemical Hazards Chemist Edson Haddad 2016 Sao Paulo – SP – Brazil Safety with Chemicals • Chemicals can only be safely handled if their properties, reactions and behavior in different situations are fully known. • This knowledge allows for the selection of the appropriate PPE –Personal Protective Equipment, as well as the techniques to be employed for containment, control and environmental monitoring. Control Actions 9Neutralization, absorption, washing/dilution, soil recovery, monitoring, waste destination. I worked 20 years and had only one accident. CHEMICAL HAZARDS COFFEE WATER OXYGEN NO SUBSTANCE IS COMPLETELY FREE OF TOXIC EFFECTS TO THE BODY 12 POPs z PESTICIDES - Aldrin, dieldrin, chlordane, DDT, endrin, heptachlor, mirex, hexachlorobenzene and toxaphene; z INDUSTRIAL SUBSTANCES - PCBs (polychlorinated biphenyls) and HCB (hexachlorobenzene); z NON-INTENTIONAL SUB PRODUCTS – hexachlorobenzene; polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/PCDF), and PCBs. aldrin DDT mirex PCB Dioxins and furans 9 POPs • PESTICIDES - chlordecone, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, lindane, pentachlorobenzene; •INDUSTRIAL SUBSTANCES - hexabromobiphenyl, hexabromodiphenyl ether and heptabromodiphenyl ether, -

Workshop Report on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife
EPA/630/R-01/002 August 2001 www.epa.gov/ncea/raf Workshop Report on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife Risk Assessment Forum U.S. Environmental Protection Agency Washington, DC DISCLAIMER This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii CONTENTS CONTRIBUTORS TO WORKSHOP DESIGN AND REPORT ........................ iv 1. INTRODUCTION ..........................................................1 2. OBJECTIVES AND DESIGN OF THE EPA/DOI WORKSHOP ......................4 3. WORKSHOP FINDINGS.....................................................6 4. CONCLUSIONS OF THE EPA/DOI WORKSHOP PLANNING GROUP ..............11 5. REFERENCES............................................................18 APPENDICES: A. Workshop Case Study: A Preliminary Problem Formulation for a Retrospective Ecological Risk Assessment Scenario.................................................... A-1 B. Workshop Case Study: A Preliminary Problem Formulation for a Prospective Ecological Risk Assessment Scenario.....................................................B-1 C. Report from the Workshop on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife.................................................... C-i I. Introduction ........................................................C-1 II. Opening Presentations...............................................C-2 -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Hexachlorobenzene UNITED NATIONS
UNITED NATIONS RC UNEP/FAO/RC/CRC.5/10/Add.1 Distr.: General 2 December 2008 United Nations English only Environment Programme Food and Agriculture Organization of the United Nations Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade Chemical Review Committee Fifth meeting Rome, 23–27 March 2009 Item 4 (b) (vii) of the provisional agenda* Listing of chemicals in Annex III to the Rotterdam Convention: review of notifications of final regulatory actions to ban or severely restrict a chemical: hexachlorobenzene Hexachlorobenzene Note by the Secretariat Addendum Supporting documentation provided by Canada The Secretariat has the honour to provide, in the annex to the present note, documentation received from Canada to support its notification of final regulatory action on hexachlorobenzene as an industrial chemical. * UNEP/FAO/RC/CRC.5/1. K0842870 161208 For reasons of economy, this document is printed in a limited number. Delegates are kindly requested to bring their copies to meetings and not to request additional copies. UNEP/FAO/RC/CRC.5/10/Add.1 Annex • Hexachlorobenzene: Priority Substances List Assessment Report, Environment Canada, Health Canada. • Environmental Assessments of Priority Substances Under the Canadian Environmental Protection Act, Guidance Manual, Version 1.0 — March 1997. Environment Canada. • SOR/2005-41; CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999; Prohibition of Certain Toxic Substances Regulations, 2005, Canada Government Gazette. 2 Canadian -

Version, Februari 4Th, 2000 Dutch Target and Intervention
www.esdat.net Esdat Environmental Database Management Software +61 2 9232 8080 Version, februari 4th, 2000 Dutch Target and Intervention Values, 2000 (the New Dutch List) ANNEXES Circular on target values and intervention values for soil remediation Four annexes belong to this circular: · annex A deals with the target values, the soil remediation intervention values and the indicative levels for serious contamination; · annex B contains the measurement and analysis regulations for soil/sediment and groundwater for the substances listed in annex A; · annex C gives the data required for determining the remediation urgency and the remediation deadline for the substances in part A; · annex D provides a guideline for dealing with substances for which there are no standards. Circular on target values and intervention values for soil remediation page 1 of 51 www.esdat.net Esdat Environmental Database Management Software +61 2 9232 8080 ANNEX A: TARGET VALUES, SOIL REMEDIATION INTERVENTION VALUES AND INDICATIVE LEVELS FOR SERIOUS CONTAMINATION Introduction Soil remediation policy uses soil remediation intervention values, indicative levels for serious contamination and target values. These three types of standards are dealt with below. The point of departure in setting standards for environmental policy as a whole is the risks involved. This strategy is set forth in the document Premises for Risk Management [Omgaan met risico’s]. The risk-based approach in environmental policy (Ministry of Housing, Spatial Planning and the Environment (VROM), Lower House of Parliament, parliamentary proceedings 1988-1989, 21 137, no. 5). The intervention values and the accompanying target values for soil/sediment and groundwater are given in table 1. -

Chlorobenzenes (PDF)
EPA-454/R-93-044 LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF CHLOROBENZENES (REVISED) Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 March 1994 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Any mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. ii CONTENTS Section Page DISCLAIMER ..................................................... ii LIST OF FIGURES .................................................. vi LIST OF TABLES .................................................. vii 1.0 PURPOSE OF DOCUMENT ..................................... 1-1 1.1 Reference for Section 1.0 ................................... 1-5 2.0 OVERVIEW OF DOCUMENT CONTENTS .......................... 2-1 2.1 References for Section 2.0 .................................. 2-5 3.0 BACKGROUND .............................................. 3-1 3.1 Nature of Pollutant ....................................... 3-1 3.1.1 Properties of Chlorobenzenes ........................... 3-5 3.1.2 Properties of Monochlorobenzene ........................ 3-6 3.1.3 Properties of Dichlorobenzenes .......................... 3-6 3.1.4 Properties of Trichlorobenzenes ......................... 3-7 3.1.5 Properties of Hexachlorobenzene ........................ 3-7 3.2 Overview of Production and Use .............................. 3-8 3.3 -

Managing Diseases and Insects in Home Orchards J
Managing diseases and insects in home orchards J. W. Pscheidt, H. Stoven, A. Thompson, B. Edmunds, N. Wiman, and R. Hilton In this guide, you can learn best pest management practices for your home Contents orchards. Suggested materials and times of application should have activity Table 1. Home garden/small orchard on the indicated pest. There are many fungicides and insecticides that are products ........................ 2 Importance of controlling diseases effective for managing the diseases and insects listed on the label when used and insects in commercial fruit according to the label directions. For more information, see the PNW Pest districts ......................... 3 Management Handbooks, at https://pnwhandbooks.org. Applying pesticides safely ......... 3 The best way to manage diseases and insects in your orchard is to combine Managing diseases and insects methods. Along with using pesticides, there are cultural and biological without using pesticides .......... 4 Apples .......................... 5 practices also that can help prevent or manage diseases and insects (see Pears ........................... 7 page 4). Pesticide timing and thorough spray coverage are the keys to good Peaches and Nectarines .......... 9 pest management. For good coverage, wet the leaves, twigs, and branches Apricots ........................10 thoroughly. (Note: This can be difficult with hand sprayers.) When you Cherries ........................11 use wettable powders, be sure to shake or stir the spray mix often during Prunes and Plums ...............13 application because the powders tend to settle at the bottom of the spray Walnuts ........................14 container after mixing. Hazelnuts (Filberts) .............14 To avoid excess chemical residues, be sure to use the correct rate and Moss and lichen .................15 proper interval between the last spray and harvest, as shown on the label. -

August, 1988 CHLOROTHALONIL Health Advisory Office of Drinking
August, 1988 CHLOROTHALONIL Health Advisory Office of Drinking Water U.S. Environmental Protection Agency I. INTRODUCTION The Health Advisory (HA) Program, sponsored by the Office of Drinking Water (ODW), provides information on the health effects, analytical methodology and treatment technology that would be useful in dealing with the contamination of drinking water. Health Advisories describe nonregulatory concentrations of drinking water contaminants at which adverse health effects would not be anticipated to occur over specific exposure durations. Health Advisories contain a margin of safety to protect sensitive members of the population. Health Advisories serve as informal technical guidance to assist Federal, State and local officials responsible for protecting public health when emergency spills or contamination situations occur. They are not to be construed as legally enforceable Federal standards. The HAs are subject to change as new information becomes available. Health Advisories are developed for one-day, ten-day, longer-term (approximately 7 years, or 10% of an individual’s lifetime) and lifetime exposures based on data describing noncarcinogenic end points of toxicity. For those substances that are known or probable human carcinogens, according to the Agency classification scheme (Group A or B), Lifetime HAs are not recommended. The chemical concentration values for Group A or B carcinogens are correlated with carcinogenic risk estimates by employing a cancer potency (unit risk) value together with assumptions for lifetime exposure and the consumption of drinking water. The cancer unit risk is usually derived from the linear multistage model with 95% upper confidence limits. This provides a low-dose estimate of cancer risk to humans that is considered unlikely to pose a carcinogenic risk in excess of the stated values. -

Reference Guide to Non-Combustion Technologies for Remediation of Persistent Organic Pollutants in Soil, Second Edition – 2010
Reference Guide to Non-combustion TechnologiesTechnologies for Remediation of Persistent Organic PollutantsPollutants in Soil, SecondS Edition - 2010 ts B an ii ta oa llu ac ll cc o u P m iic u n lla a t g tii rr o O n O tt g a yc llii n n ll c y attii on n e na orra d tt sso ap a ev ss e d B ii ss nd ee a ss udd nn ii r iitt u tiioo oo r tt siit HighHiHig latitudes e ll a a os m e -- po iidd ep DepositionDDe > evaporation PP e a M dd a ff ff o gg o oo nn t r t ii ss r High mobility oo High mobility ff ii ee pp ss cc cc ee nn aa gg aa rr Relatively high mobility rr Long-range Relatively high mobility n t Long-range n t t t aa uu i r cc i r i oceanic i oceanic o - - o rr o o gg ee transport Low mobility n transport n n n h h S o S o p p L L s s o o m m m m m m m m t t t t t t t t t a a a a a a Low latitudes t t Deposition > evaporation c Deposition > evaporation c ee ff ff ee ”” gg iinn p pp op sh as Grr ll ““G a iic T m h e e h n e C iio rm ll-C tt a a a ll iic ad ys ra h eg P De B iiore ogy med hnoll diiatiion Phytotech RR eemme giieess eddiiaattiioonn TTeecchhnnoolloog Solid Waste EPA 542-R-09-007 and Emergency Response September 2010 (5203P) www.clu-in.org/POPs Reference Guide to Non-combustion Technologies for Remediation of Persistent Organic Pollutants in Soil, Second Edition – 2010 Internet Address (URL) http://www.epa.gov Recycled/Recyclable.