Reducing Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Production of Cyclohexane Through Catalytic Hydrogenation of Benzene
Production of Cyclohexane through Catalytic Hydrogenation of Benzene Background Cyclohexane is industrially produced from Benzene as it is not a naturally available resource. Cyclohexane undergoes oxidation reactions yielding Cyclohexanone and Cyclohexanol which are precursors for the production of Adipic acid and Caprolactum. Caprolactum is the raw material used for producing polymer Nylon-6. Benzene reacts with a mixture of hydrogen and methane in contact with a Nickel based catalyst producing Cyclohexane. The conversion of this vapour phase reaction is almost 99%. Reaction involved: Benzene + Hydrogen Cyclohexane (Vapour Phase) Reactor Used: Catalytic Packed Bed Conversion Reactor Reactor conditions: Outlet Temperature = 497 K, Pressure Drop = 1.02 atm Catalyst Used: Nickel Based Process Description Fresh benzene (370 kmol/h) and excess hydrogen (1470 kmol/h) is preheated to a temperature of 422 K and sent to a packed bed reactor. A vapour phase reaction occurs in the reactor at 497 K which converts benzene to cyclohexane through catalytic hydrogenation of benzene. The conversion of this reaction is about 99%. The reactor products are cooled to 370 K and sent through a pressure reduction valve which reduces the pressure of the stream from 30 atm to 24 atm. A two stage separator separates the product cyclohexane from unreacted hydrogen and methane- first at a high pressure (24 atm) and then at a lower pressure (3 atm). The unreacted hydrogen-methane mixture is recovered from the top of the flash column and is sent to a splitter having a splittling ratio of 9:1. The smaller stream is sent as a recycle stream and mixes with fresh hydrogen, while the rest is drawn out as fuel gas for incinerators. -

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
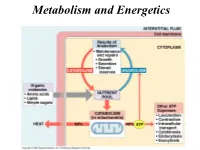
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Herbert Charles Brown, a Biographical Memoir
NATIONAL ACADEMY OF SCIENCES H E R B E R T Ch ARLES BROWN 1 9 1 2 — 2 0 0 4 A Biographical Memoir by E I-I CH I N EGIS HI Any opinions expressed in this memoir are those of the author and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 2008 NATIONAL ACADEMY OF SCIENCES WASHINGTON, D.C. Photograph Credit Here. HERBERT CHARLES BROWN May 22, 1912–December 19, 2004 BY EI -ICH I NEGISHI ERBERT CHARLES BROWN, R. B. Wetherill Research Profes- Hsor Emeritus of Purdue University and one of the truly pioneering giants in the field of organic-organometallic chemistry, died of a heart attack on December 19, 2004, at age 92. As it so happened, this author visited him at his home to discuss with him an urgent chemistry-related matter only about 10 hours before his death. For his age he appeared well, showing no sign of his sudden death the next morn- ing. His wife, Sarah Baylen Brown, 89, followed him on May 29, 2005. They were survived by their only child, Charles A. Brown of Hitachi Ltd. and his family. H. C. Brown shared the Nobel Prize in Chemistry in 1979 with G. Wittig of Heidelberg, Germany. Their pioneering explorations of boron chemistry and phosphorus chemistry, respectively, were recognized. Aside from several biochemists, including V. du Vigneaud in 1955, H. C. Brown was only the second American organic chemist to win a Nobel Prize behind R. B. Woodward, in 1965. His several most significant contribu- tions in the area of boron chemistry include (1) codiscovery of sodium borohyride (1972[1], pp. -

Appendix F. Glossary
Appendix F. Glossary 2DEG 2-dimensional electron gas A/D Analog to digital AAAR American Association for Aerosol Research ADC Analog-digital converter AEM Analytical electron microscopy AFM Atomic force microscope/microscopy AFOSR Air Force Office of Scientific Research AIST (Japan) Agency of Industrial Science and Technology AIST (Japan, MITI) Agency of Industrial Science and Technology AMLCD Active matrix liquid crystal display AMM Amorphous microporous mixed (oxides) AMO Atomic, molecular, and optical AMR Anisotropic magnetoresistance ARO (U.S.) Army Research Office ARPES Angle-resolved photoelectron spectroscopy ASET (Japan) Association of Super-Advanced Electronics Technologies ASTC Australia Science and Technology Council ATP (Japan) Angstrom Technology Partnership ATP Adenosine triphosphate B Magnetic flux density B/H loop Closed figure showing B (magnetic flux density) compared to H (magnetic field strength) in a magnetizable material—also called hysteresis loop bcc Body-centered cubic BMBF (Germany) Ministry of Education, Science, Research, and Technology (formerly called BMFT) BOD-FF Bond-order-dependent force field BRITE/EURAM Basic Research of Industrial Technologies for Europe, European Research on Advanced Materials program CAD Computer-assisted design CAIBE Chemically assisted ion beam etching CBE Chemical beam epitaxy 327 328 Appendix F. Glossary CBED Convergent beam electron diffraction cermet Ceramic/metal composite CIP Cold isostatic press CMOS Complementary metal-oxide semiconductor CMP Chemical mechanical polishing -

Chapter 3 CHEMICAL MODIFICATION of OILS and FATS
Chapter 3 CHEMICAL MODIFICATION OF OILS AND FATS From the fats and oils obtained from natural resources, the majority of them are used directly or just after refinement. While the others are used after modification by chemical process. This chapter lists some typical modifications of fats and oils by chemical means. 3-1 Alkaline Hydrolysis Figure 3-1-1: Alkaline hydrolysis (saponification) of oil to make soaps. There are many methods for hydrolysis of triacylglycerol molecule. The most common method is alkaline hydrolysis. Heating (around 100˚C)triacylglycerols with aqueous solu- tion of sodium hydroxide results in glycerol and alkaline salt of fatty acid (i.e. soap) (Figure 3-1-1). This is called saponification, and used for production of soap. 3-2 Hydrogenation Number of double bonds in oils and fats affects physical property such as melting point, crystallinity. Generally, double bonds reduce the oil’s melting point. Therefore, oils rich in unsaturated fatty acids are liquid, while ones with small amount of unsaturated fatty acids are solid or semi-solid. Hydrogenation is a process to add hydrogen atoms into double bonds of unsaturated fatty acids (Figure 3-2-1). As the result of hydrogenation, liquid oil becomes solid or semi-solid. A typical example of hydrogenation is in the process of margarine and shortening production. Vegetable oil is hydrogenated with gaseous H2 in the presence of a metal catalyst (usually nickel catalyst). If the hydrogenation is completely performed, all the double bounds are 19 Figure 3-2-1: Hydrogenation. converted to the saturated ones with the same carbon number. -

Sodium Borohydride
Version 6.6 SAFETY DATA SHEET Revision Date 01/21/2021 Print Date 09/25/2021 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Sodium borohydride Product Number : 452882 Brand : Aldrich CAS-No. : 16940-66-2 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Synthesis of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) Chemicals which, in contact with water, emit flammable gases (Category 1), H260 Acute toxicity, Oral (Category 3), H301 Skin corrosion (Category 1B), H314 Serious eye damage (Category 1), H318 Reproductive toxicity (Category 1B), H360 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 GHS Label elements, including precautionary statements Pictogram Signal word Danger Aldrich - 452882 Page 1 of 9 The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the US and Canada Hazard statement(s) H260 In contact with water releases flammable gases which may ignite spontaneously. H301 Toxic if swallowed. H314 Causes severe skin burns and eye damage. H360 May damage fertility or the unborn child. -

Elementary Iodine-Doped Activated Carbon As an Oxidizing Agent for the Treatment of Arsenic-Enriched Drinking Water
water Article Elementary Iodine-Doped Activated Carbon as an Oxidizing Agent for the Treatment of Arsenic-Enriched Drinking Water Fabio Spaziani 1,2,*, Yuli Natori 1, Yoshiaki Kinase 1, Tomohiko Kawakami 1 and Katsuyoshi Tatenuma 1 1 KAKEN Inc., Mito Institute 1044 Hori, Mito, Ibaraki 310-0903, Japan 2 ENEA Casaccia, Via Anguillarese 301, 00123 Roma, Italy * Correspondence: [email protected] Received: 18 July 2019; Accepted: 23 August 2019; Published: 27 August 2019 Abstract: An activated carbon impregnated with elementary iodine (I2), named IodAC, characterized by oxidation capability, was developed and applied to oxidize arsenite, As(III), to arsenate, As(V), in arsenic-rich waters. Batch and column experiments were conducted to test the oxidation ability of the material. Comparisons with the oxidizing agents usually used in arsenic treatment systems were also conducted. In addition, the material has been tested coupled with an iron-based arsenic sorbent, in order to verify its suitability for the dearsenication of drinking waters. IodAC exhibited a high and lasting oxidation potential, since the column tests executed on water spiked with 50 mg/L of arsenic (100% arsenite) showed that 1 cc of IodAC (30 wt% I2) can oxidize about 25 mg of As(III) (0.33 mmol) before showing a dwindling in the oxidation ability. Moreover, an improvement of the arsenic sorption capability of the tested sorbent was also proved. The results confirmed that IodAC is suitable for implementation in water dearsenication plants, in place of the commonly used oxidizing agents, such as sodium hypochlorite or potassium permanganate, and in association with arsenic sorbents. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride. -

Hydrogenation Reaction
SOP: How to Run an Atmospheric-Pressure Hydrogenation Reaction Hazards: Hydrogenation reactions pose a significant fire hazard due to the use of flammable reagents and solvents. Such reagents include palladium on carbon (Pd/C), which is highly flammable and can ignite solvents and hydrogen. It is especially dangerous after having been used for the hydrogenation. The presence of hydrogen gas increases the risk of explosion. Special Precautions: Remove any excess clutter and any flammable solvents that are not needed from your fume hood for the reaction. Be prepared for the possibility of a small fire. Do not panic if this occurs, but simply cover the flask or funnel in which there is a fire with a watch glass and it will go out. Have a suitable sized watch glass on hand. Recommended Apparatus: A three-necked flask equipped with a magnetic stirring bar, a nitrogen inlet adapter connected to a nitrogen/vacuum manifold, a glass stopper or rubber septum, and a gas inlet adapter with a stopcock and a balloon filled with hydrogen. Procedure: 1. Put a weighed quantity of the catalyst in the flask. 2. Evacuate and back-fill the flask with nitrogen 3 times. 3. Add your solvent under a countercurrent of nitrogen. CAUTION: Do not pour your solvent from a 4-liter bottle or a 1-liter bottle. Use a small Erlenmeyer flask (for example 125 mL) containing only the needed amount of solvent. 4. Add your substrate to be hydrogenated to the flask. 5. Evacuate and back-fill the flask with hydrogen. 6. If needed, you may replace the balloon with a full one as needed during the reaction. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Comparative Study of Oxidants in Removal of Chemical Oxygen Demand from the Wastewater (IJIRST/ Volume 4 / Issue 1/ 011)
IJIRST –International Journal for Innovative Research in Science & Technology| Volume 4 | Issue 1 | June 2017 ISSN (online): 2349-6010 Comparative Study of Oxidants in Removal of Chemical Oxygen Demand from the Wastewater Prof Dr K N Sheth Kavita V Italia Director PG Student Geetanjali Institute of Technical Studies, Udaipur Department of Environmental Engineering Institute of Science & Technology for Advanced Studies & Research, Vallabh Vidyanagar Abstract Chemical Oxygen Demand (COD) test involves chemical oxidation using chromic acid as a strong oxidizer. COD of a wastewater sample is the amount of oxidant- potassium dichromate that reacts with the sample when it is heated for 2 hours under controlled environmental conditions and result is expressed as mg of oxidant consumed per liter of a given sample of wastewater. COD can be helpful in determining the quantity required for dilution needed for conducting Biochemical Oxygen Demand (BOD) five day test. In the present investigation attempts have been made to use other oxidants like hydrogen peroxide H2O2, sodium hypo-chlorite, calcium hypo-chlorite and potassium permanganate for measurement of COD by standard method and compare the results obtained using potassium dichromate as strong oxidant. Experiments were carried out for each oxidizing agent for COD removal at temperatures like 1000C, 750C and 500C. The duration of exposure time in each experimental set up was taken as temperature wise, for 1000C, it was 1-5 minutes , for 750C it was 20-40 minutes and for 500C it was 45-75 minutes. It was found that temperature plays very important role in in deciding the exposure time to be allocated for reduction of COD.