(12) Patent Application Publication (10) Pub. No.: US 2010/0228065 A1 Cheung Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phosphorus-Containing Amino Acids with a P–C Bond in the Side Chain Or a P–O, P–Sorp–N Bond: Cite This: RSC Adv., 2020, 10, 6678 from Synthesis to Applications
RSC Advances View Article Online REVIEW View Journal | View Issue Phosphorus-containing amino acids with a P–C bond in the side chain or a P–O, P–SorP–N bond: Cite this: RSC Adv., 2020, 10, 6678 from synthesis to applications a b b Mathieu Arribat, Florine Cavelier * and Emmanuelle Remond´ * Since the discovery of (L)-phosphinothricin in the year 1970, the development of a-amino acids bearing a phosphorus group has been of renewed interest due to their diverse applications, including their use in [18F]-fluorolabeling, as fluorescent probes, as protecting groups and in the reversible immobilization of amino acids or peptide derivatives on carbon nanomaterials. Considerable progress has also been achieved in the field of antiviral agents, through the development of phosphoramidate prodrugs, which increase significantly the intracellular delivery of nucleoside monophosphate and monophosphonate analogues. This review aims to summarize the strategies reported in the literature for the synthesis of P(III), P(IV) and P(V) phosphorus-containing amino acids with P–C, P–O, P–SorP–N bonds in the side Received 2nd December 2019 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. chains and their related applications, including their use in natural products, ligands for asymmetric Accepted 22nd January 2020 catalysis, peptidomimetics, therapeutic agents, chemical reagents, markers and nanomaterials. The DOI: 10.1039/c9ra10917j discussion is organized according to the position of the phosphorus atom linkage to the amino acid side rsc.li/rsc-advances chain, either in an a-, b-, g-ord-position or to a hydroxyl, thiol or amino group. 1. Introduction phosphinothricin has engendered the development of numerous drugs for neurodegenerative disease treatment. -

Some Transition Metal Complexes of Trivalent Phosphorus Esters
AN ABSTRACT OF THE THESIS OF HARRY VAUGHN STUDER for the MASTER OF SCIENCE (Name) (Degree) inCHEMISTRY (Inorganic) presented on ///( (Major) / (Date') Title: SOME TRANSITION METAL COMPLEXES OF TRIVALENT PHOSPHORUS ESTERS Redacted for Privacy Abstract approved: Dr. In T. Yoke r The ligands in the series EtnP(OEt)3_11, n = 0 - 3,all form pseudotetrahedral high-spin bis -complexes with cobalt (II) chloride. Magnetic, spectrophotometric, conductivity, and molecular weight data show that the phosphorus ester ligands (but not the phosphine) also form five-coordinate low-spin tris-complexes; these can be isolated with the phosphonite and phosphinite ligands, while the existence of the tris -phosphite is marginal.At cobalt (II) to phosphorus ester mole ratios of 1:2, a mixture of species is present in all cases.All are non-conducting in nitrobenzene. The ester complexes are much more susceptible to autoxidation than is the phosphine complex. Some Transition Metal Complexes of Trivalent Phosphorus Esters by Harry Vaughn Studer A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science June 1972 APPROVED: Redacted for Privacy Profes of Chemi str y / in crge of major Redacted for Privacy Head of Department of Chemistry Redacted for Privacy Dean of Graduate School Date thesis is presented Typed by Donna Olson f arry Vatighn Studer ACKNOWLEDGEMENTS The author wishes to thank his research advisor, Dr. John T. Yoke, for his expert direction of this research, for his patience, and for the opportunity of learning through association with him. Acknowledgement is made to the donors of the Petroleum Re- search Fund, administered by the American Chemical Society, for the support of this work. -

Metal Complexes As Catalysts for the Synthesis of Heterocycles
METAL COMPLEXES AS CATALYSTS FOR THE SYNTHESIS OF HETEROCYCLES Steven Lal Department of Chemistry, Imperial College London A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, Imperial College London May 2013 1 Declaration I certify that all the work in this thesis is solely my own, except where explicitly stated and appropriately referenced. No part of the thesis has been submitted previously for a degree at this, or any other, university. 2 Copyright Notice Imperial College of Science, Technology and Medicine Department Of Chemistry Metal Complexes as Catalysts for the Synthesis of Heterocycles © 2013 Steven Lal [email protected] The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives License. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the license terms of this work. Steven Lal Department of Chemistry Imperial College London Exhibition Road London SW7 2AZ UK www.imperial.ac.uk 3 Contents Abstract ...................................................................................................................................... 8 Acknowledgements ................................................................................................................. -

Advanced Studies on the Synthesis of Organophosphorus Compounds
ALMA MATER STUDIORUM UNIVERSITÀ DI BOLOGNA DOTTORATO DI RICERCA IN SCIENZE CHIMICHE (XIX° ciclo) Area 03 Scienze Chimiche-CHIM/06 Chimica Organica Dipartimento di Chimica Organica “A. Mangini” Coordinatore: Chiar.mo Prof. Vincenzo Balzani Advanced Studies on the Synthesis of Organophosphorus Compounds Dissertazione Finale Presentata da: Relatore: Dott. ssa Marzia Mazzacurati Prof. Graziano Baccolini Co-relatore: Dott.ssa Carla Boga INDEX Index: Keywords…………………………………………………………….………….VII Chapter 1…………………………………………………………………………..3 GENERAL INTRODUCTION ON PHOSPHORUS CHEMISTRY 1.1 Organophosphorus Chemistry………………………………………………….4 1.1.1 Phosphines………………………………………………………………..5 1.1.2 Phosphonates……………………………………………………………..6 1.1.3 Phosphites………………………………………………………………...7 1.2 Uses of Organophosphorus Compounds………………………………………..7 1.2.1 Agricultural Application………………………………………………….8 1.2.2 Catalysis……………………………………………………………..…....9 1.2.3 Organophosphorus Conpounds in Medicine…………………………….11 1.2.4 Phosphorus in Biological Compounds…………………………………..12 1.3 References……………………………………………………………………..15 Chapter 2…………………………………………………………………………17 THE HYPERCOORDINATE STATES OF PHOSPHORUS 2.1 The 5-Coordinate State of Phosphorus……………………………………….17 2.2 Pentacoordinated structures and their non rigid character…………………….18 2.3 Permutational isomerization…………………………………………………..19 2.3.1 Berry pseudorotation……………………………………………………20 2.3.2 Turnstile rotation………………………………………………………..21 2.4 The 6-Coordinate State of Phosphorus……………………………………….22 2.5 References…………………………………………………………………......24 I Chapter -

Organophosphorus Chemistry (Kanda, 2019)
Baran lab Group Meeting Yuzuru Kanda Organophosphorus Chemistry 09/20/19 bonding and non-bonding MOs of PH3 bonding and non-bonding MOs of PH5 # of R P(III) ← → P(V) O P P R R R R R R phosphine phosphine oxide D3h C3v C2v O O JACS. 1972, 3047. P P P P R NH R OH R OH R NH Chem. Rev. 1994, 1339. R 2 R R R 2 D C phosphineamine phosphinite phosphinate phosphinamide 3h 4v O O O R P R P R P R P R P R P NH2 NH2 OH OH NH2 NH2 H2N HO HO HO HO H2N phosphinediamine phosphonamidite phosphonite phosphonate phosphonamidate phosphonamide O O O O H N P P P P P P P P 2 NH HO HO HO HO HO HO H2N H N 2 NH2 NH2 OH OH NH2 NH2 NH2 2 H2N HO HO HO HO H2N H2N phosphinetriamine phosphorodiamidite phosphoramidite phosphite phosphate phosphoramidate phosphorodiamidate phosphoramide more N O more O Useful Resources more N P P P Corbridge, D. E. C. Phosphorus: Chemistry, Biochemistry and H OH H H H H H H H Technology, 6th ed.; CRC Press Majoral, J. P. New Aspects In Phosphorus Chemistry III.; Springer phosphinous phosphane phosphane Murphy, P. J. Organophosphorus Reagents.; Oxford acid oxide Hartley, F. R. The chemistry of organophosphorus compounds, O O volume 1-3.; Wiley P P P Cadogan. J. I. G. Organophosphorus Reagents in Organic H OH H OH H OH HO HO H Synthesis.; Academic Pr phosphonate phosphonus acid phosphinate Not Going to Cover ↔ (phosphite) Related GMs Metal complexes, FLP, OPV Highlights in Peptide and Protein NH S R • oxidation state +5, +4, +3, +2, +1, 0, -1, -2, -3 Synthesis (Malins, 2016) R P-Stereogenic Compounds P P R P • traditionally both +3 and -3 are written as (III) R • 13/25th most abundant element on the earth (Rosen, 2014) R • but extremely rare outside of our solar system Ligands in Transition Metal phosphine imide phosphine sulfide phosphorane Catalysis (Farmer, 2016) Baran lab Group Meeting Yuzuru Kanda Organophosphorus Chemistry 09/20/19 Me P Me Me Low-Coordinate Low Oxidation State P tBu tBu P P phosphaalkyne R N P PivCl 2 P Me Cl TMS OTMS NaOH R = tBu Nb N tBu PTMS3 P O H O P NR2 then Na/Hg tBu N -2 Nb tBu R2N Me Nb N tBu 5x10 mbar, 160 ºC NR2 95% R2N Me N O 1. -

Acscatal.0C01923.Pdf
This is a repository copy of Phosphoranyl Radical Fragmentation Reactions Driven by Photoredox Catalysis. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/163811/ Version: Published Version Article: Rossi-Ashton, James A., Clarke, Aimee K., Unsworth, William P. orcid.org/0000-0002- 9169-5156 et al. (1 more author) (2020) Phosphoranyl Radical Fragmentation Reactions Driven by Photoredox Catalysis. ACS Catalysis. pp. 7250-7261. ISSN 2155-5435 https://doi.org/10.1021/acscatal.0c01923 Reuse This article is distributed under the terms of the Creative Commons Attribution (CC BY) licence. This licence allows you to distribute, remix, tweak, and build upon the work, even commercially, as long as you credit the authors for the original work. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ This is an open access article published under a Creative Commons Attribution (CC-BY) License, which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. pubs.acs.org/acscatalysis Perspective Phosphoranyl Radical Fragmentation Reactions Driven by Photoredox Catalysis James A. Rossi-Ashton,* Aimee K. Clarke, William P. Unsworth, and Richard J. K. Taylor Cite This: ACS Catal. 2020, 10, 7250−7261 Read Online ACCESS Metrics & More Article Recommendations ABSTRACT: Photocatalytic generation of phosphoranyl radicals is fast emerging as an essential method for the generation of diverse and valuable radicals, typically via deoxygenation or desulfurization processes. -

(12) United States Patent (10) Patent No.: US 9.221,727 B2 Cheung Et Al
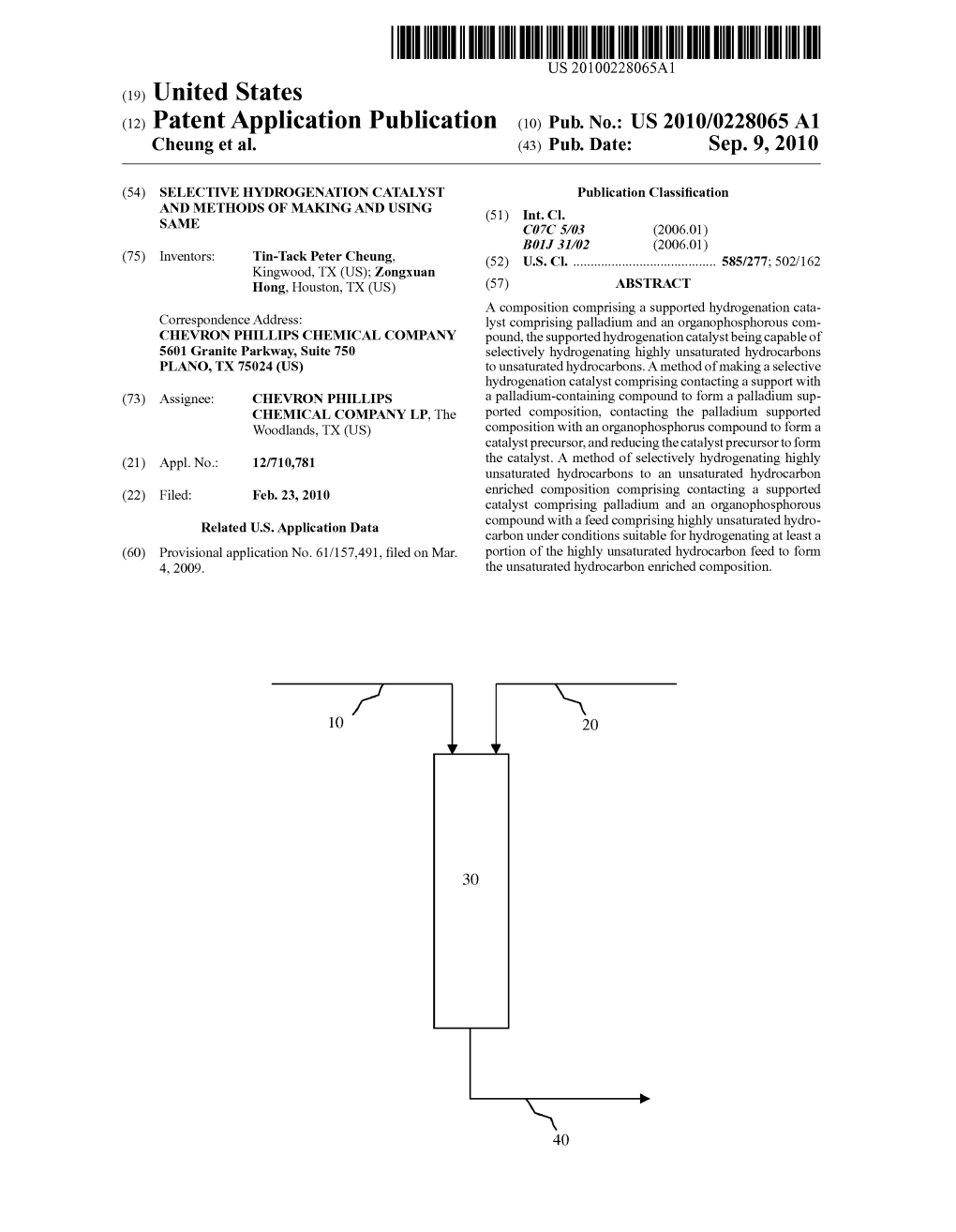
US009221727B2 (12) United States Patent (10) Patent No.: US 9.221,727 B2 Cheung et al. (45) Date of Patent: Dec. 29, 2015 (54) SELECTIVE HYDROGENATION CATALYST USPC .......... 585/269,277: 502/162, 164, 262,327, AND METHODS OF MAKING AND USING 502/328,330, 333,339, 439 SAME See application file for complete search history. (56) References Cited (71) Applicant: Chevron Phillips Chemical Company LP, The Woodlands, TX (US) U.S. PATENT DOCUMENTS (72) Inventors: Tin-Tack Peter Cheung, Kingwood, TX 3,463,830 A 8/1969 Dunning et al. (US); Zongxuan Hong, Houston, TX 3,625,755 A 12, 1971 Potrafike (US) (Continued) (73) Assignee: Chevron Phillips Chemical Company FOREIGN PATENT DOCUMENTS LP, The Woodlands, TX (US) CN 1422695 A 6, 2003 (*) Notice: Subject to any disclaimer, the term of this CN 1425,637 A 6, 2003 patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS (21) Appl. No.: 14/669,541 Boitiaux, J.P. et al., “Hydrogenation of highly unsaturated hydrocar bons over highly dispersed Pd catalyst. Part II: Ligand effect of (22) Filed: Mar. 26, 2015 piperidine.” Applied Catalysis, 1985, vol. 15, pp. 317-326, Elsevier Science Publishers B.V., Amsterdam, XPOO2577931. (65) Prior Publication Data (Continued) US 2015/O197464 A1 Jul. 16, 2015 Primary Examiner — Cam N. Nguyen (74) Attorney, Agent, or Firm — Conley Rose, P.C.; Rodney Related U.S. Application Data B. Carroll; Lynda Jolly (62) Division of application No. 14/134,282, filed on Dec. (57) ABSTRACT 19, 2013, now Pat. No. 9,012,348, which is a division A composition comprising a Supported hydrogenation cata of application No. -

Measuring the Electronic Effect of Some Phosphines and Phosphites
Measuring the electronic effect of some phosphines and phosphites By Sanaa Moftah Ali Abushhewa A dissertation submitted to the University of Manchester for the degree of Master of Science by research in the Faculty of Engineering and Physical Sciences. School of Chemistry 2010 Contents page Abstract 6 Declaration 7 Acknowledgement 8 1. Introduction 9 1.1 History of Phosphorus 9 1.2 Phosphorus (III) compounds 10 1.3 Preparation and Properties of Phosphines 11 1.4 Preparation and Properties of Phosphites 12 1.5 Bonding of phosphorus (III) ligands 14 1.6 Measurement of Electronic and Steric Effects 15 1.7 Steric parameter 17 1.8 Electronic parameter 17 1.9 Effects of electronegative substituents 21 1.10 Phosphines and Phosphites ligand effects 21 2. Experimental section 24 General conditions 25 2.1- Preparation of phosphites 25 2.1.1- Synthesis of 2, 2’- Biphenyl Chlorophosphite 25 2.1.2- Synthesis of 1H,1H,2H,2H-perfluorodecyldiphenyl phosphinite 25 2 2.1.3- Synthesis of 3-fluorophenyldiphenyl phosphinite 26 2.1.4- Synthesis of 2-fluorophenyldiphenyl phosphinite 26 2.1.5- Synthesis of 2, 2-biphenyltrifluoroethyl phosphinite 27 2.1.6- Synthesis of 2, 2'-biphenylhexafluoroisopropyl phosphinite 27 2.1.7-Synthesis of 2, 2'-biphenylpropyl phosphite 28 2.2-Preparation of P(III) Selenides 28 2.2.1- Synthesis of triphenylphosphine selenide 28 2.2.2- Synthesis of triphenylphosphite selenide 29 2.2.3- Synthesis of tris(2,4,6-trimethylphenyl)phosphite selenide 29 2.2.4- Synthesis of diphenyl(o-tolyl) phosphine selenide 30 2.2.5- Synthesis of 2, 2’-biphenyl -

Chemistry of Phosphorylated Formaldehyde Derivatives. Part I
Molecules 2014, 19, 12949-13009; doi:10.3390/molecules190912949 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Chemistry of Phosphorylated Formaldehyde Derivatives. Part I Vasily P. Morgalyuk Laboratory of Organophosphorus Compounds, Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilova str., 28, Moscow 119991, Russia; E-Mail: [email protected]; Tel.: +7-499-135-92-50; Fax: +7-495-135-65-49 Received: 8 July 2014; in revised form: 8 August 2014 / Accepted: 15 August 2014 / Published: 25 August 2014 Abstract: The underinvestigated derivatives of unstable phosphorylated formaldehyde acetals and some of the structurally related compounds, such as thioacetals, aminonitriles, aminomethylphosphinoyl compounds, are considered. Separately considered are halogen aminals of phosphorylated formaldehyde, acetals of phosphorylated formaldehyde of H-phosphinate-type and a phosphorylated gem-diol of formaldehyde. Synthetic methods, chemical properties and examples of practical applications are given. Keywords: phosphorylated acetals; -thioacetals; -aminonitriles; -aminomethylphosphinoyl compounds; -chloro(or bromo)aminals; -gem-dioles; -ketene acetale; H-phosphinate 1. Introduction Among organophosphorus compounds, α-phosphorylated carbonyl compounds stand out by the capacity of cleavage of phosphorus–carbon bond under mild conditions when reacted with nucleophiles [1–5]. The cleavage of phosphorus–carbon bond may proceed spontaneously as well. At the same time, α-oxoalkylphosphinoyl compounds also retain properties inherent in carbonyl compounds, for example, they undergo cross aldol condensation. The least stable compounds among them are phosphorylated formaldehyde derivatives, dialkyl formylphosphonates (1) [6–11] and N,N,N',N'-tetraalkyl formylphosphondiamides (2) [12] (Figure 1), whose existence was even disputed in the first half of the 1970s [13,14]. -

Synthesis, Purification, and Characterization of Tetraphosphine Ligands Deniz Cevik
Synthesis, purification, and characterization of tetraphosphine ligands Deniz Cevik To cite this version: Deniz Cevik. Synthesis, purification, and characterization of tetraphosphine ligands. Other. Univer- sité Paris-Saclay, 2017. English. NNT : 2017SACLX026. tel-01631221 HAL Id: tel-01631221 https://pastel.archives-ouvertes.fr/tel-01631221 Submitted on 8 Nov 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2017SACLX026 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY PREPAREE A ÉCOLE POLYTECHNIQUE ECOLE DOCTORALE N° 517 2MIB | Sciences chimiques : Molécules, matériaux, instrumentation et biosystèmes Spécialité de doctorat : Chimie Par Mme. Deniz Çevik Synthesis, Purification, and Characterization of Tetraphosphine Ligands Thèse présentée et soutenue à Palaiseau, le 17. Juillet 2017 : Composition du Jury : Mme. Hii, King Kuok (Mimi) Professeur Imperial College London Rapporteure M. Manoury, Eric DR - CNRS au LCC (Toulouse) Rapporteur M. Voituriez, Arnaud DR - CNRS á l’ICSN Président M. van Leeuwen, Piet Chaire d’Attractivité au LPCNO, INSA-Toulouse -

20. Phosphorus Chemistry
Professor David L. Van Vranken Chemistry 201: Organic Reaction Mechanisms I Topic 20: Phosphorus Chemistry References: Literature cited Phosphorus Functional Groups ■ Here’s how to name phosphorus functional groups: R R R OR R OR O O R P P P R P P(III) R R OR O R phosphine phosphinite phosphonite phosphite (ester) O O O O R R R OR R OR O O P P P R P R P(V) R R OR O R phosphine oxide phosphinate phosphonate phosphate (ester) ■ Few of these functional groups are common. O O O H P N P HO OH O CH HO F 3 glyphosate sarin Phosphorus Functional Groups ■ pKas reveal the difference in basicity between trialkylphosphines and triarylphosphines Et Ph .. + + N N Ph2N Et Et Ph Ph resonance H H reduces l.p. basicity pKa 11 ~ -5 .. Bu Ph Ph P + + 2 P P not so much Bu Bu Ph Ph H H resonance pKa 9 3 ■ Trialkylphosphines are way more nucleophilic than triarylphosphines Bu Ph Bu P: Ph P: Bu Ph way more nucleophilic Howard ST, et al. Can. J. Chem. 1997, 75, 60. Allman, T.; Goel, R. G. Can. J. Chem. 1982, 60, 716.. POCl3 is a Very Reactive Electrophile ■ Old conditions for elimination of OH groups. Contrast the outcomes. SOCl Cl 2 pyridine OH POCl3 pyridine (solvent) ■ Phosphates don’t substitute very quickly, but Cl3P=O is special ■ Vilsmeier-Haack Reaction - amides attack Cl3P=O + NMe O Cl NMe 2 2 E.A.S. MeO + MeO H2O MeO H OMe OMe workup OMe POCl3 + DMF Mechanism for formation of the Vilsmeier reagent: : . -

(12) United States Patent (10) Patent No.: US 9,365,793 B2 Boffa (45) Date of Patent: *Jun
US0093.65793B2 (12) United States Patent (10) Patent No.: US 9,365,793 B2 Boffa (45) Date of Patent: *Jun. 14, 2016 (54) METHODS AND COMPOSITIONS FOR (56) References Cited REDUCING WEAR IN INTERNAL COMBUSTON ENGINESLUBRICATED U.S. PATENT DOCUMENTS WITH A LOW PHOSPHOROUS CONTENT 3,997.454 A 12/1976 Adams BORATE-CONTAINING LUBRICATING OL 3,997.457 A 12/1976 Takahashi et al. 4,089,790 A 5, 1978 Adams 4,163,729 A 8, 1979 Adams (71) Applicant: Alexander B. Boffa, Richmond, CA 4,431,552 A 2f1984 Salentine (US) 4472.288 A 9/1984 Frost, Jr. 4.495,075 A 1/1985 Buckley (72) Inventor: Alexander B. Boffa, Richmond, CA 4,534,873. A 8, 1985 Clark 4,717,490 A 1/1988 Salentine (US) 4,975,096 A 12/1990 Buckley, III 5,635,459 A 6/1997 Stoffa et al. (73) Assignee: Chevron Oronite Company LLC, San 5,962,377 A 10/1999 Baumgartet al. Raman, CA (US) 6,306,802 B1 10/2001 Shaub 6,534.450 B1 3/2003 Harrison et al. (*) Notice: Subject to any disclaimer, the term of this 6,569,818 B2 5/2003 Nakazato et al. 6,632,781 B2 10/2003 Harrison et al. patent is extended or adjusted under 35 6,696,393 B1 2, 2004 Boffa U.S.C. 154(b) by 0 days. 6,730,638 B2 5/2004 Farng et al. 6,737,387 B2 5, 2004 Harrison et al. This patent is Subject to a terminal dis 6,777,378 B2 8/2004 Abraham et al.