Clam Dissection Handout
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Marine Bivalve Molluscs
Marine Bivalve Molluscs Marine Bivalve Molluscs Second Edition Elizabeth Gosling This edition first published 2015 © 2015 by John Wiley & Sons, Ltd First edition published 2003 © Fishing News Books, a division of Blackwell Publishing Registered Office John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030‐5774, USA For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley‐blackwell. The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. Limit of Liability/Disclaimer of Warranty: While the publisher and author(s) have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -
Field Identification Guide to the Living Marine Resources In
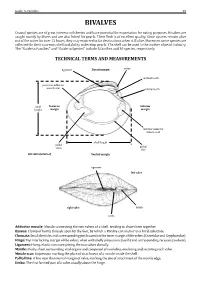
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

Freshwater Mussels of the Pacific Northwest
Freshwater Mussels of the Pacifi c Northwest Ethan Nedeau, Allan K. Smith, and Jen Stone Freshwater Mussels of the Pacifi c Northwest CONTENTS Part One: Introduction to Mussels..................1 What Are Freshwater Mussels?...................2 Life History..............................................3 Habitat..................................................5 Role in Ecosystems....................................6 Diversity and Distribution............................9 Conservation and Management................11 Searching for Mussels.............................13 Part Two: Field Guide................................15 Key Terms.............................................16 Identifi cation Key....................................17 Floaters: Genus Anodonta.......................19 California Floater...................................24 Winged Floater.....................................26 Oregon Floater......................................28 Western Floater.....................................30 Yukon Floater........................................32 Western Pearlshell.................................34 Western Ridged Mussel..........................38 Introduced Bivalves................................41 Selected Readings.................................43 www.watertenders.org AUTHORS Ethan Nedeau, biodrawversity, www.biodrawversity.com Allan K. Smith, Pacifi c Northwest Native Freshwater Mussel Workgroup Jen Stone, U.S. Fish and Wildlife Service, Columbia River Fisheries Program Offi ce, Vancouver, WA ACKNOWLEDGEMENTS Illustrations, -

Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Transposed Hinge Structures in Lamellibranches
TRANSACTIONS OF THE • SAN DIEGO SOCIETY OF NATURAL HISTORY ,/ VoLUME VII, No. 26, pp. 299-318, plate 19 • TRANSPOSED HINGE STRUCTURES IN LAMELLIBRANCHS BY P. PoPENOE AND FINDLAY W. W. A. • Balch Graduate School of the Geological Sciences California Institute of Technology Pasadena, California SAN DIEGO, CALIFORNIA PRINTED FOR THE SOCIETY OCTOBER 6, 1933 • • COMMITTEE ON PUBLICATION U.S. GRANT, IV, Chairman FRED BAKER CLINTON G. ABBOTT, Editor • TRANSPOSED HINGE STRUCTURES IN LAMELLIBRANCHS 1 BY W. P. PoPENOE AND W. A. FINDLAY California Institute of Technology INTRODUCTION In the course of study of a collection of Eocene fossils from Claiborne Bluff, Alabama, the senior author of tl1is paper noticed two valves, one right and one left, of the lamellibranch Venericardia parva Lea, in whicl1 the dentition is partially transposed. Subsequently, the authors made an examination of more than five thousand lamellibranch valves, representit1g both recent and fossil shells, in search of further exa1nples of hinge-trans position. We have found a total number of twenty-six valves exhibiting this variation. Study of these specimens has revealed some hitherto unreported facts regarding the principles of hinge-transposition. Therefore in this paper, we shall describe and discuss these specimens, and shall present such con clusions as seem justified by the data assembled. Citations to the literature are made by author, date, and page, referring to the list at the end of the paper. DEFINITION OF T ERMS A transposed lamellibranch hinge is defined as one that exhibits in the right valve the hinge ele1nents normally occurring in the left valve, and vice-versa. -

Mechanics Unlocks the Morphogenetic Puzzle of Interlocking Bivalved Shells
Mechanics unlocks the morphogenetic puzzle of interlocking bivalved shells Derek E. Moultona,1 , Alain Gorielya , and Regis´ Chiratb aMathematical Institute, University of Oxford, Oxford, OX2 6GG, United Kingdom; and bCNRS 5276, LGL-TPE (Le Laboratoire de Geologie´ de Lyon: Terre, Planetes,` Environnement), Universite´ Lyon 1, 69622 Villeurbanne Cedex, France Edited by Sean H. Rice, Texas Tech University, Lubbock, TX, and accepted by Editorial Board Member David Jablonski November 11, 2019 (received for review September 24, 2019) Brachiopods and mollusks are 2 shell-bearing phyla that diverged tal events causing shell injuries. Yet, in all cases the interlocking from a common shell-less ancestor more than 540 million years ago. of the 2 shell edges is tightly maintained. These observations Brachiopods and bivalve mollusks have also convergently evolved imply that the interlocking pattern emerges as the result of epi- a bivalved shell that displays an apparently mundane, yet strik- genetic interactions modulating the behavior of the secreting ing feature from a developmental point of view: When the shell mantle during shell development. is closed, the 2 valve edges meet each other in a commissure that Here, we provide a geometric and mechanical explanation forms a continuum with no gaps or overlaps despite the fact that for this morphological trait based on a detailed analysis of the each valve, secreted by 2 mantle lobes, may present antisymmet- shell geometry during growth and the physical interaction of the ric ornamental patterns of varying regularity and size. Interlock- shell-secreting soft mantle with both the rigid shell edge and ing is maintained throughout the entirety of development, even the opposing mantle lobe. -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Atlas of the Freshwater Mussels (Unionidae)
1 Atlas of the Freshwater Mussels (Unionidae) (Class Bivalvia: Order Unionoida) Recorded at the Old Woman Creek National Estuarine Research Reserve & State Nature Preserve, Ohio and surrounding watersheds by Robert A. Krebs Department of Biological, Geological and Environmental Sciences Cleveland State University Cleveland, Ohio, USA 44115 September 2015 (Revised from 2009) 2 Atlas of the Freshwater Mussels (Unionidae) (Class Bivalvia: Order Unionoida) Recorded at the Old Woman Creek National Estuarine Research Reserve & State Nature Preserve, Ohio, and surrounding watersheds Acknowledgements I thank Dr. David Klarer for providing the stimulus for this project and Kristin Arend for a thorough review of the present revision. The Old Woman Creek National Estuarine Research Reserve provided housing and some equipment for local surveys while research support was provided by a Research Experiences for Undergraduates award from NSF (DBI 0243878) to B. Michael Walton, by an NOAA fellowship (NA07NOS4200018), and by an EFFRD award from Cleveland State University. Numerous students were instrumental in different aspects of the surveys: Mark Lyons, Trevor Prescott, Erin Steiner, Cal Borden, Louie Rundo, and John Hook. Specimens were collected under Ohio Scientific Collecting Permits 194 (2006), 141 (2007), and 11-101 (2008). The Old Woman Creek National Estuarine Research Reserve in Ohio is part of the National Estuarine Research Reserve System (NERRS), established by section 315 of the Coastal Zone Management Act, as amended. Additional information on these preserves and programs is available from the Estuarine Reserves Division, Office for Coastal Management, National Oceanic and Atmospheric Administration, U. S. Department of Commerce, 1305 East West Highway, Silver Spring, MD 20910. -

Download Complete Work
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Laseron, C. F., 1956. A revision of the New South Wales Leptonidae (Mollusca: Pelecypoda). Records of the Australian Museum 24(2): 7–22. [23 November 1956]. doi:10.3853/j.0067-1975.24.1956.640 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia 7 A REVISION OF THE NEW SOUTH WALES LEPTONIDAE (MOLLUSCA: Pelecypoda) (l!"igs. 1-27) By CHARLES F. LASERON, F.R.Z.S. (This research has been assisted by a gTant from tbe Science and Industry Endowment Fund.) INTRODUCTION. Th~ group of bivalves dealt with in this paper has been classified differently by AustralasIan cO~lChologists. Hedley in his check list, 1918, used Leptonidae as a family name.. Powell In "~~ellfish of New Zealand", 1937, divided the group into two families Lasa81dae and Erycll1ldae. Ootton and Godfrey, "The Mollusca of South Australia", 1938, used Leptonacea as a superfamily, divided into two families, Leptonidae and Montacutidae. POivell again, in a second edition, 1946, reverted to the single family Leptonidae . .Th.at arrangement is followed here. The group, whether considered as a family or superfamlly, seems a natural one, and the characters, both anatomical and of the shell, are reasonably definable. Many of the genera are nestling, others are reputecl to be either commensal or parasitic, but the latter habits have Hot been 1l0ticeil in any of the Peronian forms.