The Third-Millennium International System of Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Linear and Angular Velocity Examples
Linear and Angular Velocity Examples Example 1 Determine the angular displacement in radians of 6.5 revolutions. Round to the nearest tenth. Each revolution equals 2 radians. For 6.5 revolutions, the number of radians is 6.5 2 or 13 . 13 radians equals about 40.8 radians. Example 2 Determine the angular velocity if 4.8 revolutions are completed in 4 seconds. Round to the nearest tenth. The angular displacement is 4.8 2 or 9.6 radians. = t 9.6 = = 9.6 , t = 4 4 7.539822369 Use a calculator. The angular velocity is about 7.5 radians per second. Example 3 AMUSEMENT PARK Jack climbs on a horse that is 12 feet from the center of a merry-go-round. 1 The merry-go-round makes 3 rotations per minute. Determine Jack’s angular velocity in radians 4 per second. Round to the nearest hundredth. 1 The merry-go-round makes 3 or 3.25 revolutions per minute. Convert revolutions per minute to radians 4 per second. 3.25 revolutions 1 minute 2 radians 0.3403392041 radian per second 1 minute 60 seconds 1 revolution Jack has an angular velocity of about 0.34 radian per second. Example 4 Determine the linear velocity of a point rotating at an angular velocity of 12 radians per second at a distance of 8 centimeters from the center of the rotating object. Round to the nearest tenth. v = r v = (8)(12 ) r = 8, = 12 v 301.5928947 Use a calculator. The linear velocity is about 301.6 centimeters per second. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

TECHNICAL Nbte D-626
NASA TN D-626 LIBRARY COPY NOV 16 1960 SPACE FLIGHT LANGLEY FIELD, VIRGINIA t TECHNICAL NbTE D-626 SATELLITE ATTDUDE CONTROL USING A COMBINATION OF INERTIA WHEELS AND A BAR MAGNET By James J. Adams and Roy F. Brissenden Langley Research Center Langley Field, Va. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION WASHINGTON November 1960 . NATIONAL AERONAUTICS AND SPACE ADMINISTRATION TECHNICAL NOTE D-626 SATELLITE ATTITUDE CONTROL USING A COMBINATION OF INERTIA WHEELS AND A BAR MAGNET By James J. Adams and Roy F. Brissenden SUMMARY The use of inertia wheels to control the attitude of a satellite has currently aroused much interest. The stability of such a system has been studied in this investigation. A single-degree-of-freedom analysis indicates that a response with suitable dynamic characteristics and pre- cise control can be achieved by commanding the angular velocity of the inertia wheel with an error signal that is the sum of the attitude error, the attitude rate, and the integral of the attitude error. A digital computer was used to study the three-degree-of-freedom response to step displacements, and the results indicate that the cross-coupling effects of inertia coupling and precession coupling had no effect on system sta- bility. A study was also made of the use of a bar magnet to supplement the inertia wheels by providing a means of removing any momentum intro- duced into the system by disturbances such as aerodynamic torques. A study of a case with large aerodynamic torques, with a typical orbit, indicated that the magnet was a suitable device for supplying the essen- tial trimming force. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Some Considerations on Quantities of Dimension One in Oscillatory Motions
Some considerations on quantities of dimension one in oscillatory motions Leonardo Gariboldi Dipartimento di Fisica, Università degli Studi di Milano, Via Celoria 16, 20133, Milano, Italy. E-mail: [email protected] (Received 13 February 2012, accepted 27 May 2012) Abstract The habit of not writing a multiplicative factor of dimension one leads to equations, which, though dimensionally correct, can pose some problems to most students as for the analysis of the units of measurement of the written physical quantities. The proposed analysis of the derivation of the equations for a simple oscillator and a forced oscillator with damping shows how to handle with the units of measurement. Similar considerations can be done for the formula of error propagation for oscillating functions. Keywords: Units of measurement, Radian, Oscillatory motion. Resumen El hábito de no escribir un factor multiplicativo de una dimensión conduce a las ecuaciones, las cuales, aunque de dimensiones correctas, pueden plantear algunos problemas a la mayoría de los estudiantes como para el análisis de las unidades de medida de las cantidades físicas escritas. El análisis propuesto de la derivación de las ecuaciones para un oscilador simple y un oscilador forzado con amortiguamiento muestra cómo controlar a las unidades de medida. Se pueden hacer consideraciones similares para la fórmula de propagación de errores para funciones oscilantes. Palabras clave: Unidades de medición, Radián, Movimiento oscilatorio. PACS: 01.40.Fk, 01.40.gb, O6.20.Dk ISSN 1870-9095 I. INTRODUCTION II. A PROBLEM WITH THE UNIT OF MEASUREMENT OF THE ANGULAR The study of oscillatory motions in the most common FREQUENCY physics textbooks used in undergraduate courses (see, e.g., [1, 2, 3, 4, 5]) is based on definitions and laws described in The frequency ν of an oscillatory motion is defined as the previous chapters (e.g.: Cinematic, Newton’s laws) and on number of oscillation cycles per time. -

Technical Standards Board Standard
TECHNICAL REV. STANDARDS TSB 003 MAY1999 BOARD Issued 1965-06 400 Commonwealth Drive, Warrendale, PA 15096-0001 STANDARD Revised 1999-05 Superseding TSB 003 JUN92 Submitted for recognition as an American National Standard Rules for SAE Use of SI (Metric) Units Foreword—SI denotes The International System of Units (Le Système International d’Unités). SI was established in 1960, under the Treaty of the Meter, by Resolutions and Recommendations of the General Conference on Weights and Measures (Conférence Générale des Poids et Mesures, CGPM) and the International Committee for Weights and Measures (Comité International des Poids et Mesures, CIPM) on The International System of Units. The abbreviation "SI" is used in all languages. In 1969, the SAE Board of Directors issued a directive that "SAE will include SI units in SAE Standards and other technical reports." During the ensuing several decades, SAE metric policy evolved and implementation progressed. The SAE’s current metric policy is, "Operating Boards shall not use any weights and measures system other than metric (SI), except when conversion is not practical, or where a conflicting world industry practice exists." Principal driving forces for SAE metrication were: worldwide movement to metric units; enactment of United States Federal metric legislation and the resultant national metrication activity; the international trend in industry and business throughout the world, and the growing international scope of SAE. Currently, the widespread, strong support for international standards harmonization is another key motivating factor in the global metrication movement. TSB 003 (formerly SAE J916) has been updated periodically, to reflect SAE metric policy evolution—as well as developments in the specific, formal content of SI; and in the correct, consistent usage and application of SI...which sometimes is referred to as "the modern version of the metric measurement system." The content of TSB 003 is consistent with international and U.S. -

STANDARD ABBREVIATIONS���� Index No
circ. Circumference A Area or Amperes Ckt. Circuit F Fill, Farad J Joule AAA American Automobile Association Cl. or Clear Clearance F or Final Final Quantity JB Junction Box AASHO American Association Of State Highway Officials CL, C/L or | Center Line F & I Furnish & Install Jct. Junction AASHTO American Association Of State Highway And Transportation Officials CM Concrete Monument F to F Face to Face Jt. Joint ABC Asphalt Base Course CMB Concrete Median Barrier FA Federal Aid or Fine Aggregate K Design Hour Factor or Kelvin Abd. Abandoned CMP Corrugated Metal Pipe FAC Florida Administrative Code k Kilo (prefix) ABS Acrylonitrite-Butadiene-Styrene Pipe CMPA Corrugated Metal Pipe Arch FAP Federal Aid Project kg Kilogram AC, Ac. Acre Co. County or Company FC Friction Course kg/m Kilogram Per Meter AC or Asph. Conc. Asphaltic Concrete Col. Column FD French Drain kg/m 2 Kilogram Per Square Meter Accel. Acceleration Com. Commercial or Common Fdn. Foundation kg/m 3 Kilogram Per Cubic Meter Act. Actuated COMM Committee or By Committee FDOT Florida Department Of Transportation Kilo One Thousand ADA The Americans With Disabilities Act Comp. Composite FE Floor Elevation Kip 1000 Pounds Adh. Adhesive Con. Connect or Connection Fed. Federal km Kilometer Adj. Adjust Conc. Concrete Fert. Fertilizer km/h Kilometer Per Hour ADT Average Daily Traffic Const. Construct or Construction FES Flared End Section kn Knot AADT Annual Average Daily Traffic Contrl. Controller FETS Flared End Terminal Section kN Kilonewton Agg. Aggregate Cont. Continuation FH Fire Hydrant kPa Kilopascal Ah. Ahead Contr. Contractor FHWA Federal Highway Administration ksi Kips Per Square Inch AISC American Institute Of Steel Construction Coord. -

Linear and Angular Velocity Angular Displacement
Lesson 6.2 Linear and Angular Velocity Angular Displacement As a circular object rotates counterclockwise about its center, an object at the edge moves through an angle relative to its starting position known as the angular displacement, or angle of rotation. y θ A x 0 B Example 1 Determine the angular displacement in radians of 6.5 revolutions. Round to the nearest tenth. Each revolution equals 2 radians. For 6.5 revolutions, the number of radians is 6.5 2 or 13 . 13 radians equals about 40.8 radians. Angular Velocity The ratio of change in a central angle to the time required for the change is known as angular velocity. Angular velocity is represented by the lowercase Greek letter ω (omega). y θ θ ω A x t 0 Where θ is the angular B displacement in radians Example 2 Determine the angular velocity if 4.8 revolutions are completed in 4 seconds. Round to the nearest tenth. The angular displacement is 4.8 2 or 9.6 radians. θ 9.6 ω t 4 7.539822369 Use a calculator. The angular velocity is about 7.5 radians per second. Dimensional Analysis In dimensional analysis, unit labels are treated as mathematical factors and can be DIVIDED OUT. Example 3 AMUSEMENT PARK Jack climbs on a horse that is 12 feet from the center of a merry-go-round. The merry-go-round makes 3 ¼ rotations per minute. Determine Jack’s angular velocity in radians per second. Round to the nearest hundredth. The merry-go-round makes 3 ¼ or 3.25 revolutions per minute. -
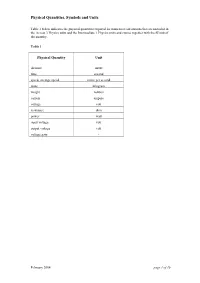
Quantity Symbol Sheet
Physical Quantities, Symbols and Units Table 1 below indicates the physical quantities required for numerical calculations that are included in the Access 3 Physics units and the Intermediate 1 Physics units and course together with the SI unit of the quantity. Table 1 Physical Quantity Unit distance metre time second speed, average speed metre per second mass kilogram weight newton current ampere voltage volt resistance ohm power watt input voltage volt output voltage volt voltage gain - February 2004 page 1 of 10 Physical Quantities, Symbols and Units Table 2 below indicates the physical quantities required for numerical calculations that are included in the Standard Grade Physics course together with: • the symbol used by SQA • the SI unit of the quantity (and alternative units included in the course) • the abbreviation for the unit used in Credit level examinations. In General level examinations full words are used for the units of all physical quantities. Table 2 Physical Quantity Symbol Unit Unit Abbreviation distance d or s metre m light year height h metre m wavelength λ metre m amplitude Α metre m time t second s speed, final speed v metre per second m/s initial speed u metre per second m/s change of speed ∆v metre per second m/s average speed v metre per second m/s frequency f hertz Hz acceleration a metre per second per second m/s2 acceleration due to gravity g metre per second per second m/s2 gravitational field strength g newton per kilogram N/kg mass m kilogram kg weight W newton N force, thrust F newton N energy E joule -
National Advisory Commitiee for Aeronautics
mCD r--------------------------------------------------- O":l N Z f-i ~ u NATIONAL ADVISORY COMMITIEE ~ Z FOR AERONAUTICS TECHNICAL NOTE 2985 A FLIGHT INVESTIGATION OF THE EFFECT OF STEADY HOLLING ON THE NATURAL FREQUENCIES OF A BODY- TAIL COMBINATION By Norman R . Bergrun and Paul A. Nickel Ames Aeronautical Laboratory Moffett Field, Calif. Washington August 1953 ERRATA NACA TN 2985 A FLIGHT INVESTIGATION OF THE EFFECT OF STEADY ROLLING ON THE NATURAL FREQUENCIES OF A BODY-TAIL COMBINATION By Norman R. Bergrun and Paul A. Nickel August, 1953 1. In figure 8, sublegend 8 (a ) should read IIshort-period oscillationll and sublegend 8(b) should read IIlong-period oscillation. 1I Iy I z 2 . I n the title of figure 9, -- = -- = 7 instead of 1/7. SC Sc J lY NATIONAL ADVISORY COMMITTEE FOR AERONAUTICS TECHNICAL NOTE 2985 A FLIGm' INVESTIGATION OF THE EFFECT OF STEADY ROLLINc:J. ON THE NATURAL FREQUENCIES OF A BODY-TAIL COMBINATION By Norman R. Bergrun and Paul A. Nickel SUMMARY Flight experiments with a freely falling model have been conducted to observe the effects of steady rolling on the pitching and yawing motions of a body-tail combination. Time histories of the pitching and yawing motions show that steady rolling introduces into the pitch oscil lations the yawing motions, and into the yaw oscillations, the pitching motions. The observed effects of steady rolling on the values of fre quencies are compared with those predicted by theory for damping and for no damping. When damping is included, the calculated frequencies are in good agreement with the observed frequencies, although a good first-order approximation of the frequencies may be obtained by neglect ing damping. -

Formulas for Motorized Linear Motion Systems
FORMULAS FOR MOTORIZED LINEAR MOTION SYSTEMS Haydon Kerk Motion Solutions : 203 756 7441 Pittman Motors : 267 933 2105 www.HaydonKerkPittman.com 2 Symbols andSYMBOLS Units AND UNITS Symbol Description Units Symbol Description Units a linear acceleration m/s2 P power W 2 a angular acceleration rad/s Pavg average power W 2 ain angular acceleration rad/s PCV power required at constant velocity W angular acceleration 2 power required at system input aout rad/s Pin W α temperature coefficient /°C Ploss dissipated power W DV viscous damping factor Nm s/rad Pmax maximum power W F force N Pmax(f) maximum power (final“hot”) W Fa linear force required during acceleration (FJ +Ff +Fg) N Pmax(i) maximum power (initial “cold”) W Ff linear force required to overcome friction N Pout output power W Fg linear force required to overcome gravity N PPK peak power W FJ linear force required to overcome load inertia N Rm motor regulation RPM/Nm 2 2 g gravitational constant (9.8 m/s ) m/s Rmt motor terminal resistance Ω I current A Rmt(f) motor terminal resistance (final“hot”) Ω ILR locked rotor current A Rmt(i) motor terminal resistance (initial “cold”) Ω IO no-load current A Rth thermal resistance °C/W IPK peak current A r radius m IRMS RMS current A S linear distance m J inertia Kg-m2 t time s inertia of belt Kg-m2 torque Nm JB T inertia of the gear box Kg-m2 T torque required to overcome load inertia Nm JGB a reflected inertia at system input Kg-m2 T torque required at motor shaft during acceleration Jin a (motor) Nm 2 continuous rated motor torque Jout -

5 Angular Velocity – Some Final Points 19
Physics #19: The Kinematics of Circular Motion Adam Kirsch Andrea Hayes Larry Ottman Brenda Meery Bradley Hughes Mara Landers Art Fortgang Lori Jordan CK12 Editor James H Dann, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) www.ck12.org AUTHORS Adam Kirsch To access a customizable version of this book, as well as other Andrea Hayes interactive content, visit www.ck12.org Larry Ottman Brenda Meery Bradley Hughes Mara Landers Art Fortgang CK-12 Foundation is a non-profit organization with a mission to Lori Jordan reduce the cost of textbook materials for the K-12 market both CK12 Editor in the U.S. and worldwide. Using an open-content, web-based James H Dann, Ph.D. collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational CONTRIBUTORS content that will serve both as core text as well as provide an Chris Addiego adaptive environment for learning, powered through the FlexBook Ari Holtzman Platform®. Antonio De Jesus López Tinyen Shih Copyright © 2012 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms.