Recurrent Keratoconus in a Patient with Leber Congenital Amaurosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
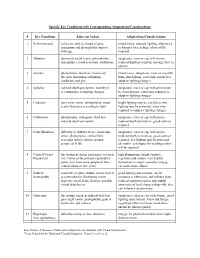
Specific Eye Conditions with Corresponding Adaptations/Considerations
Specific Eye Conditions with Corresponding Adaptations/Considerations # Eye Condition Effect on Vision Adaptations/Considerations 1 Achromotopsia colors are seen as shades of grey, tinted lenses, reduced lighting, alternative nystagmus and photophobia improve techniques for teaching colors will be with age required 2 Albinism decreased visual acuity, photophobia, sunglasses, visor or cap with a brim, nystagmus, central scotomas, strabismus reduced depth perception, moving close to objects 3 Aniridia photophobia, field loss, vision may tinted lenses, sunglasses, visor or cap with fluctuate depending on lighting brim, dim lighting, extra time required to conditions and glare adapt to lighting changes 4 Aphakia reduced depth perception, inability to sunglasses, visor or cap with a brim may accommodate to lighting changes be worn indoors, extra time required to adapt to lighting changes 5 Cataracts poor color vision, photophobia, visual bright lighting may be a problem, low acuity fluctuates according to light lighting may be preferred, extra time required to adapt to lighting changes 6 Colobomas photophobia, nystagmus, field loss, sunglasses, visor or cap with a brim, reduced depth perception reduced depth perception, good contrast required 7 Color Blindness difficulty or inability to see colors and sunglasses, visor or cap with a brim, detail, photophobia, central field reduced depth perception, good contrast scotomas (spotty vision), normal required, low lighting may be preferred, peripheral fields alternative techniques for teaching colors -

Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: a Literature Review and Proposal of Novel Applications
Hindawi Journal of Ophthalmology Volume 2020, Article ID 1497089, 10 pages https://doi.org/10.1155/2020/1497089 Research Article Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: A Literature Review and Proposal of Novel Applications Hiroshi Eguchi ,1 Fumika Hotta,1 Shunji Kusaka,1 and Yoshikazu Shimomura2 1Department of Ophthalmology, Kindai University, Faculty of Medicine, 377-2 Ohnohigashi, Osakasayama, Osaka 589-8511, Japan 2Department of Ophthalmology, Fuchu Eye Center, 1-10-17 Hiko-cho, Izumi, Osaka 594-0076, Japan Correspondence should be addressed to Hiroshi Eguchi; [email protected] Received 26 June 2020; Revised 12 August 2020; Accepted 21 August 2020; Published 11 September 2020 Academic Editor: Sang Beom Han Copyright © 2020 Hiroshi Eguchi et al. &is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Intraoperative optical coherence tomography (iOCT) is widely used in ophthalmic surgeries for cross-sectional imaging of ocular tissues. &e greatest advantage of iOCTis its adjunct diagnostic efficacy, which facilitates to decision-making during surgery. Since the development of microscopic-integrated iOCT (MIOCT), it has been widely used mainly for vitreoretinal and anterior segment surgeries. In corneal transplantation, MIOCT allows surgeons to visualise structure underneath the turbid and distorted cornea, which are impossible to visualise with a usual microscope. Real-time visualisation of hard-to-see area reduces the operation time and leads to favorable surgical outcomes. &e use of MIOCT is advantageous for a variety of corneal surgical procedures. Here, we have reviewed articles focusing on the utility of iOCT and MIOCTin penetrating keratoplasty, deep anterior lamellar keratoplasty, Descemet stripping automated endothelial keratoplasty, and Descemet membrane endothelial keratoplasty. -

A Patient & Parent Guide to Strabismus Surgery
A Patient & Parent Guide to Strabismus Surgery By George R. Beauchamp, M.D. Paul R. Mitchell, M.D. Table of Contents: Part I: Background Information 1. Basic Anatomy and Functions of the Extra-ocular Muscles 2. What is Strabismus? 3. What Causes Strabismus? 4. What are the Signs and Symptoms of Strabismus? 5. Why is Strabismus Surgery Performed? Part II: Making a Decision 6. What are the Options in Strabismus Treatment? 7. The Preoperative Consultation 8. Choosing Your Surgeon 9. Risks, Benefits, Limitations and Alternatives to Surgery 10. How is Strabismus Surgery Performed? 11. Timing of Surgery Part III: What to Expect Around the Time of Surgery 12. Before Surgery 13. During Surgery 14. After Surgery 15. What are the Potential Complications? 16. Myths About Strabismus Surgery Part IV: Additional Matters to Consider 17. About Children and Strabismus Surgery 18. About Adults and Strabismus Surgery 19. Why if May be Important to a Person to Have Strabismus Surgery (and How Much) Part V: A Parent’s Perspective on Strabismus Surgery 20. My Son’s Diagnosis and Treatment 21. Growing Up with Strabismus 22. Increasing Signs that Surgery Was Needed 23. Making the Decision to Proceed with Surgery 24. Explaining Eye Surgery to My Son 25. After Surgery Appendix Part I: Background Information Chapter 1: Basic Anatomy and Actions of the Extra-ocular Muscles The muscles that move the eye are called the extra-ocular muscles. There are six of them on each eye. They work together in pairs—complementary (or yoke) muscles pulling the eyes in the same direction(s), and opposites (or antagonists) pulling the eyes in opposite directions. -

Hyperopia Hyperopia
Hyperopia Hyperopia hyperopia hyperopia • Farsightedness, or hyperopia, • Farsightedness occurs if your eyeball is too as it is medically termed, is a short or the cornea has too little curvature, so vision condition in which distant objects are usually light entering your eye is not focused correctly. seen clearly, but close ones do • Its effect varies greatly, depending on the not come into proper focus. magnitude of hyperopia, the age of the individual, • Approximately 25% of the the status of the accommodative and general population is hyperopic (a person having hyperopia). convergence system, and the demands placed on the visual system. By Judith Lee and Gretchyn Bailey; reviewed by Dr. Vance Thompson; Flash illustration by Stephen Bagi 1. Cornea is too flap. hyperopia • In theory, hyperopia is the inability to focus and see the close objects clearly, but in practice many young hyperopics can compensate the weakness of their focusing ability by excessive use of the accommodation functions of their eyes. Hyperopia is a refractive error in • But older hyperopics are not as lucky as them. By which parallel rays of light aging, accommodation range diminishes and for 2. Axial is too short. entering the eye reach a focal older hyperopics seeing close objects becomes point behind the plane of the retina, while accommodation an impossible mission. is maintained in a state of relaxation. 1 Amplitude of Accommodation hyperopia Maximum Amplitude= 25-0.4(age) • An emmetropic eye for reading and other near Probable Amplitude= 18.5-.3(age) work, at distance of 16 in (40cm), the required amount of acc. -

Olivia Steinberg ICO Primary Care/Ocular Disease Resident American Academy of Optometry Residents Day Submission
Olivia Steinberg ICO Primary Care/Ocular Disease Resident American Academy of Optometry Residents Day Submission The use of oral doxycycline and vitamin C in the management of acute corneal hydrops: a case comparison Abstract- We compare two patients presenting to clinic with an uncommon complication of keratoconus, acute corneal hydrops. Management of the patients differs. One heals quickly, while the other has a delayed course to resolution. I. Case A a. Demographics: 40 yo AAM b. Case History i. CC: red eye, tearing, decreased VA x 1 day OS ii. POHx: (+) keratoconus OU iii. PMHx: depression, anxiety, asthma iv. Meds: Albuterol, Ziprasidone v. Scleral CL wearer for approximately 6 months OU vi. Denies any pain OS, denies previous occurrence OU, no complaints OD c. Pertinent Findings i. VA cc (CL’s)- 20/25 OD, 20/200 PH 20/60+2 OS ii. Slit Lamp 1. Inferior corneal thinning and Fleisher ring OD, central scarring OD, 2+ diffuse microcystic edema OS, Descemet’s break OS (photos and anterior segment OCT) 2. 2+ diffuse injection OS 3. D&Q A/C OU iii. Intraocular Pressures: deferred OD due to CL, 9mmHg OS (tonopen) iv. Fundus Exam- unremarkable OU II. Case B a. Demographics: 39 yo AAM b. Case History i. CC: painful, red eye, tearing, decreased VA x 1 day OS ii. POHx: unremarkable iii. PMHx: hypertension iv. Meds: unknown HTN medication v. Wears Soflens toric CL’s OU; reports previous doctor had difficulty achieving proper fit OU; denies diagnosis of keratoconus OU vi. Denies any injury OS, denies previous occurrence OU, no complaints OD c. -

Surgical Options in Managing Convergent Strabismus Fixus Related to High Myopia
Ophthalmol Ina 2015;41(2):107-115 107 Case Report Surgical Options in Managing Convergent Strabismus Fixus Related to High Myopia Jessica Zarwan, Gusti G Suardana, Anna P Bani Department of Ophthalmology, Faculty of Medicine, Indonesia University Cipto Mangunkusumo Hospital, Jakarta ABSTRACT Background: Convergent strabismus fixus is a condition in which one or both eyes are anchored in extreme adduction. This condition is caused by superotemporal herniation of the enlarged globe from the muscle cone through the space between the superior and lateral rectus muscle. It is commonly related to high myopia. Recently, transposition type procedure which unite the superior and lateral rectus muscles were proposed as the treatment of choice in this condition. However, there is no clear consensus regarding the best surgical procedure in convergent strabismus fixus. This case series reported three rare cases of convergent strabismus fixus related to high myopia and highlight the option of surgical procedure that can be done in these cases. Case Illustration: Three patients presented with convergent strabismus fixus of both eyes with history of bilateral high myopia since childhood. All patients showed Krimsky tests of >95∆ET preoperatively, with medial displacement of superior rectus and inferior displacement of lateral rectus of both eyes in all patients on orbital imaging. The bilateral Yokoma procedure both resulted in an orthophoric postoperative result with marked improvement in abduction and elevation. The hemi-Jensen also showed a significant improvement of over than 60∆ on the operated eye, resorting it to a central position during primary gaze. Conclusion: Convergent strabismus fixus is a rare condition and frequently associated with high myopia. -

Strabismus, Amblyopia & Leukocoria
Strabismus, Amblyopia & Leukocoria [ Color index: Important | Notes: F1, F2 | Extra ] EDITING FILE Objectives: ➢ Not given. Done by: Jwaher Alharbi, Farrah Mendoza. Revised by: Rawan Aldhuwayhi Resources: Slides + Notes + 434 team. NOTE: F1& F2 doctors are different, the doctor who gave F2 said she is in the exam committee so focus on her notes Amblyopia ● Definition Decrease in visual acuity of one eye without the presence of an organic cause that explains that decrease in visual acuity. He never complaints of anything and his family never noticed any abnormalities ● Incidence The most common cause of visual loss under 20 years of life (2-4% of the general population) ● How? Cortical ignorance of one eye. This will end up having a lazy eye ● binocular vision It is achieved by the use of the two eyes together so that separate and slightly dissimilar images arising in each eye are appreciated as a single image by the process of fusion. It’s importance 1. Stereopsis 2. Larger field If there is no coordination between the two eyes the person will have double vision and confusion so as a compensatory mechanism for double vision the brain will cause suppression. The visual pathway is a plastic system that continues to develop during childhood until around 6-9 years of age. During this time, the wiring between the retina and visual cortex is still developing. Any visual problem during this critical period, such as a refractive error or strabismus can mess up this developmental wiring, resulting in permanent visual loss that can't be fixed by any corrective means when they are older Why fusion may fail ? 1. -

Management of Vith Nerve Palsy-Avoiding Unnecessary Surgery
MANAGEMENT OF VITH NERVE PALSY-AVOIDING UNNECESSARY SURGERY P. RIORDAN-E VA and J. P. LEE London SUMMARY for unrecovered VIth nerve palsy must involve a trans Unresolved Vlth nerve palsy that is not adequately con position procedure3.4. The availability of botulinum toxin trolled by an abnormal head posture or prisms can be to overcome the contracture of the ipsilateral medial rectus 5 very suitably treated by surgery. It is however essential to now allows for full tendon transplantation techniques -7, differentiate partially recovered palsies, which are with the potential for greatly increased improvements in amenable to horizontal rectus surgery, from unrecovered final fields of binocular single vision, and deferment of palsies, which must be treated initially by a vertical any necessary surgery to the medial recti, which is also muscle transposition procedure. Botulinum toxin is a likely to improve the final outcome. valuable tool in making this distinction. It also facilitates This study provides definite evidence, from a large full tendon transposition in unrecovered palsies, which series of patients, of the potential functional outcome from appears to produce the best functional outcome of all the the surgical treatment of unresolved VIth nerve palsy, transposition procedures, with a reduction in the need for together with clear guidance as to the forms of surgery that further surgery. A study of the surgical management of 12 should be undertaken in specific cases. The fundamental patients with partially recovered Vlth nerve palsy and 59 role of botulinum toxin in establishing the degree of lateral patients with unrecovered palsy provides clear guidelines rectus function and hence the correct choice of initial sur on how to attain a successful functional outcome with the gery, and as an adjunct to transposition surgery for unre minimum amount of surgery. -

Painless Presentation of Acute Hydrops in Keratoconus with Serial In-Vivo Imaging
Painless Presentation of Acute Hydrops in Keratoconus with Serial In-vivo Imaging Sara Berke-Silva, O.D. and Kimberly Reed, O.D. Nova Southeastern University Abstract Acute corneal hydrops is typically associated with acute pain, photophobia, and tearing at onset, but our patient denied these symptoms. The poster will present this case and review current diagnostic and therapeutic strategies, including controversies. I. Case History a. 21 year old Hispanic female b. The patient woke up 3 hours prior to examination with an acute onset of a ‘white spot’ and reduced vision OD. Denies pain, photophobia, tearing. c. After further questioning, the patient reported foreign body sensation OU that was unchanged from the normal amount she experiences without her contact lenses. d. Diagnosed with Keratoconus at age 15. Wore corneal RGPs until two years ago when semi-scleral lenses were employed with excellent visual outcomes. II. Pertinent Findings a. The patient was not photophobic and denied pain throughout the exam. Increased lacrimation was evident, unbeknownst to the patient. b. Slit lamp examination revealed significant corneal edema centrally with an absence of an anterior chamber reaction. Epithelial bullae were present. c. OCT confirmed posterior corneal compromise and significant corneal edema throughout all layers, with several bullae and microcysts in the epithelium. III. Differential Diagnosis a. Hydrops b. Corneal abrasion caused by contact lens wear c. Corneal edema caused by contact lens overwear d. Corneal infection causing ulceration e. Acute, severe corneal edema due to other cause of endothelial failure IV. Diagnosis and Discussion a. Acute corneal hydrops in the context of keratoconus has typically been associated with pain, photophobia, and tearing at onset (1,2,3), but our patient denied having these symptoms. -

Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular
perim Ex en l & ta a l ic O p in l h Journal of Clinical & Experimental t C h f a o l m l a o Ke et al., J Clin Exp Ophthalmol 2017, 8:6 n l o r g u y o Ophthalmology J DOI: 10.4172/2155-9570.1000694 ISSN: 2155-9570 Case Report Open Access Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular Massage Following Trabeculectomy Hongmin Ke, Chengguo Zuo and Mingkai Lin* State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, 54 Xianlie Nan Road, Guangzhou, China *Corresponding author: Mingkai Lin, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, 54 Xianlie Nan Road, Guangzhou, China, 510060, E- mail: [email protected] Received date: November 13, 2017; Accepted date: November 21, 2017; Published date: November 23, 2017 Copyright: © 2017 Ke H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Purpose: To report a case of acute keratoconus-like corneal hydrops in a patient with long-term ocular massage following trabeculectomy. Methods: Case report and review of medical literature. Results: A rare complication of acute keratoconus-like corneal hydrops occurred in a patient following the use of ocular massage to maintain satisfactory aqueous humor filtration after trabeculectomy. The patient had a history of high myopia but denied previous ocular trauma, allergic disease and a family history of keratoconus. Slit-lamp examination demonstrated keratoconus-like corneal hydrops with formation of epithelial microcystic, and intrastromal cleft. -

Associations Between Hyperopia and Other Vision and Refractive Error Characteristics
1040-5488/14/9104-0383/0 VOL. 91, NO. 4, PP. 383Y389 OPTOMETRY AND VISION SCIENCE Copyright * 2014 American Academy of Optometry ORIGINAL ARTICLE Associations between Hyperopia and Other Vision and Refractive Error Characteristics Marjean Taylor Kulp*, Gui-shuang Ying†, Jiayan Huang‡, Maureen Maguire†, Graham Quinn§, Elise B. Ciner||, Lynn A. Cyert**, Deborah A. Orel-Bixler††, and Bruce D. Moore||, for the VIP Study Group ABSTRACT Purpose. To investigate the association of hyperopia greater than +3.25 diopters (D) with amblyopia, strabismus, aniso- metropia, astigmatism, and reduced stereoacuity in preschoolers. Methods. Three- to five-year-old Head Start preschoolers (N = 4040) underwent vision examination including monocular visual acuity (VA), cover testing, and cycloplegic refraction during the Vision in Preschoolers Study. Visualacuity was tested with habitual correction and was retested with full cycloplegic correction when VAwas reduced below age norms in the presence of significant refractive error. Stereoacuity testing (Stereo Smile II) was performed on 2898 children during study years 2 and 3. Hyperopia was classified into three levels of severity (based on the most positive meridian on cycloplegic refraction): group 1: greater than or equal to +5.00 D, group 2: greater than +3.25 D to less than +5.00 D with interocular difference in spherical equivalent greater than or equal to 0.50 D, and group 3: greater than +3.25 D to less than +5.00 D with interocular difference in spherical equivalent less than 0.50 D. ‘‘Without’’ hyperopia was defined as refractive error of +3.25 D or less in the most positive meridian in both eyes. -
Strabismus: a Decision Making Approach
Strabismus A Decision Making Approach Gunter K. von Noorden, M.D. Eugene M. Helveston, M.D. Strabismus: A Decision Making Approach Gunter K. von Noorden, M.D. Emeritus Professor of Ophthalmology and Pediatrics Baylor College of Medicine Houston, Texas Eugene M. Helveston, M.D. Emeritus Professor of Ophthalmology Indiana University School of Medicine Indianapolis, Indiana Published originally in English under the title: Strabismus: A Decision Making Approach. By Gunter K. von Noorden and Eugene M. Helveston Published in 1994 by Mosby-Year Book, Inc., St. Louis, MO Copyright held by Gunter K. von Noorden and Eugene M. Helveston All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior written permission from the authors. Copyright © 2010 Table of Contents Foreword Preface 1.01 Equipment for Examination of the Patient with Strabismus 1.02 History 1.03 Inspection of Patient 1.04 Sequence of Motility Examination 1.05 Does This Baby See? 1.06 Visual Acuity – Methods of Examination 1.07 Visual Acuity Testing in Infants 1.08 Primary versus Secondary Deviation 1.09 Evaluation of Monocular Movements – Ductions 1.10 Evaluation of Binocular Movements – Versions 1.11 Unilaterally Reduced Vision Associated with Orthotropia 1.12 Unilateral Decrease of Visual Acuity Associated with Heterotropia 1.13 Decentered Corneal Light Reflex 1.14 Strabismus – Generic Classification 1.15 Is Latent Strabismus